Dorzolamide/ Timolol 20mg/Ml + 5mg/Ml Eye Drops Solution
PACKAGE LEAFLET: INFORMATION FORTHE USER
Dorzolamide/Timolol 20mg/ml + 5mg/ml eye drops, solution
Dorzolamide/Timolol
(referred to as Dorzolamide/Timolol throughout this leaflet)
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or ifyou notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Dorzolamide/Timolol is and what it is used for
2. Before you use Dorzolamide/Timolol
3. Howto use Dorzolamide/Timolol
4. Possible side effects
5. Howto store Dorzolamide/Timolol
6. Further information
1. WHAT DORZOLAMIDE/TIMOLOL IS AND WHAT IT IS USED FOR
Dorzolamide/Timolol is a combination of two medicines: dorzolamide and timolol.
• Dorzolamide belongs to a group of medicines called 'carbonic anhydrase inhibitors'.
• Timolol belongs to a group of medicines called 'beta-blockers'
Dorzolamide/Timolol is prescribed to lower raised pressure within the eye in the treatment of glaucoma when beta-blocker eye drops used alone are not adequate.
2. BEFOREYOU USE DORZOLAMIDE/TIMOLOL Do not use Dorzolamide/Timolol
• ifyou are allergic (hypersensitive) to any of the ingredients of this solution,
• ifyou have respiratory problems such as asthma, have a history of asthma or have, or have had in the past severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough),
• ifyou have severe kidney problems, or a prior history of kidney stones,
• ifyou have a disturbance in the pH (acid/alkali balance) of your blood,
• ifyou have certain heart problems, including certain heart rhythm disturbances producing an abnormally slow heart rate or severe heart failure.
Ifyou think any of these apply to you, do not use Dorzolamide/Timolol until you have consulted your doctor. Take special care with Dorzolamide/Timolol Ifyou have a history of heart disease your doctor may wish to monitor your pulse rate and other signs of this disease while you are using Dorzolamide/Timolol.
Tell your doctor ifyou now have or have had liver problems, ifyou have muscle weakness or have been diagnosed as having myasthenia gravis.
You should also tell your doctor ifyou now have, or have had in the past, breathing problems, asthma or chronic obstructive pulmonary disease, Prinzmetal's angina (chest pains while resting), other heart problems (including disturbances of heart rate such as slow heart beat or severe heart failure), coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome), low blood pressure, diabetes as timolol may mask signs and symptoms of low blood sugar, overactivity of the thyroid gland as timolol may mask signs and symptoms and about any allergies to a medicine you have taken.
Ifyou develop conjunctivitis (redness and irritation of the eye[s]), swelling of the eye or eyelids, skin rash, or itching in and around the eye contact your doctor immediately. Such symptoms may be due to an allergic reaction or may be a side effect of Dorzolamide/Timolol (See'POSSIBLE SIDE EFFECTS'). Tell your doctor ifyou develop an eye infection, receive an eye injury, have eye surgery, develop other reactions or worsening ofsymptoms.
Ifyou wear soft contact lenses, it is important that your lenses are removed before using your eye drops and not put back into your eyes until 15 minutes after using your eye drops as the preservative benzalkonium chloride may possibly discolour the contact lenses.
Before you have an operation and anaesthesia (even at the dentist), tell your doctor or dentist that you are using Dorzolamide/Timolol, as there may be a sudden fall in blood-pressure associated with the anaesthetic.
Use in children
There is limited experience with Dorzolamide/Timolol in infants and children.
Use in elderly
In studies with Dorzolamide/Timolol eye drops solution the effects of Dorzolamide/Timolol eye drops solution were similar in both elderly and younger patients.
Using other medicines
Please tell your doctor or pharmacist ifyou are taking or have recently taken any other medicines, including medicines obtained without a prescription. This is particularly important if any of the following apply to you:
• You are taking antihypertensive medicines which are used to lower high blood pressure or medicines to treat heart disease such as calcium channel blockers and 13-blockers or digoxin
• You are taking quinidine (used to treat heart conditions and some types of malaria) or digoxin
• You are using another eyedrop that contains a 13-blocker
• You are taking another carbonic anhydrase inhibitor such as acetazolamide. You may be taking this type of medicine by mouth, as eye drops, or by some other method
• You are taking monoamine oxidase inhibitors (MAOIs) or selective serotonin reuptake inhibitors (SSRIs) both of which are used to treat depression or another illness
• You are taking a parasympathomimetic medicine which may have been prescribed to help you pass urine. Parasympathomimetics are also a particular type of medicine which are sometimes used to help restore normal movements through the bowel
• You are taking narcotics such as morphine used to treat moderate to severe pain or ifyou are taking large doses of aspirin. Although there is no evidence that dorzolamide hydrochloride interacts with aspirin, some other medicines which are related to dorzolamide hydrochloride and which are taken by mouth, have been known to interact with aspirin
• You are taking medicines to treat diabetes or high blood sugar
• You are taking epinephrine (adrenaline)
• You are taking antidepressants known as fluoxetine and paroxetine
• Dorzolamide/Timolol can affect or be affected by other medicines you are using, including other eye drops for the treatment ofglaucoma.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Do not use Dorzolamide/Timolol ifyou are pregnant unless your doctor considers it necessary. Tell your doctor ifyou are pregnant or planning to become pregnant.
Do not use Dorzolamide/Timolol ifyou are breast-feeding. Dorzolamide/Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding. Driving and using machines
Dorzolamide/Timolol may cause side effects such as blurred vision in some patients. Do not drive or use any tools or machines until the symptoms have cleared.
Important information about some of the ingredients of Dorzolamide/Timolol
Dorzolamide/Timolol contains the preservative benzalkonium chloride.
• Benzalkonium chloride may cause eye irritation
• Benzalkonium chloride is known to discolour soft contact lenses. Avoid contact with soft contact lenses. Remove contact lenses prior to application and wait until 15 minutes before re-insertion.
3. HOWTO USE DORZOLAMIDE/TIMOLOL
Always use Dorzolamide/Timolol exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The appropriate dosage and duration of treatment will be established by your doctor.
The usual dose is one drop in the affected eye(s) twice a day, for example in the morning and in the evening.
Ifyou use Dorzolamide/Timolol with another eye drop, leave at least 10 minutes between putting in Dorzolamide/Timolol and the other medicine.
Do not change the dosage of the drug without consulting your doctor. Ifyou must stop treatment, contact your doctor immediately.
Do not allow the tip of the container to touch your eye or areas around your eye. It may become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the container, keep the tip of the container away from contact with any surface.
In order to secure correct dosage - the dropper tip should not be enlarged.
Instructions for use:
It is recommended that you wash your hands before putting in your eye drops.
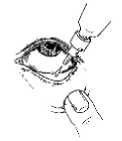
5. Repeat steps 3 & 4 with the other eye if instructed to do so by your doctor.
6. Put the cap back on and close the bottle straight after you have used it.
It may be easier to apply your eye drops in front of a mirror.
1. Before using the medication for the first time, be sure that the tamper-proof seal on the bottle neck is unbroken. A gap between the bottle and the cap is normal for an unopened bottle.
2. Take off the cap of the bottle.
3. Tilt your head back and gently pull your lower eyelid down to form a small pocket between your eyelid and your eye.
4. Turn the bottle upside down, and squeeze it until a single drop is dispensed into the eye as directed by your doctor. DO NOT TOUcH YOUR EYE OR eyelid WITH The DROPPERTIP.

After using Dorzolamide/Timolol, press a finger into the corner of your eye, by the nose for 2 minutes.
This helps to stop Dorzolamide/Timolol getting into the rest of the body.
If you use more Dorzolamide/Timolol than you should
It is important to keep to the dose your doctor has prescribed. If you put too many drops in your eye or swallow any of the contents of the bottle, you mayfeel unwell, for example you may become light-headed, have difficulty breathing, or feel that your heart rate has slowed. If you feel any of the above effects you should seek medical attention immediately.
If you forget to use Dorzolamide/Timolol
It is important to use Dorzolamide/Timolol as prescribed by your doctor.
If you miss a dose, apply it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to your regular dosing schedule.
Do not use a double dose to make up for forgotten individual doses.
If you stop taking Dorzolamide/Timolol
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Dorzolamide/Timolol can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Dorzolamide/Timolol without speaking to your doctor.
Like other medicines applied into the eyes, dorzolamide/ timolol is absorbed into the blood. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected.
Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions. This may cause similar side effects as seen with intravenous and oral beta-blocking agents.
If you develop generalised allergic reactions including hives (or itchy rash), localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction, swelling beneath the skin (that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty in breathing or swallowing), you should stop using Dorzolamide /Timolol and talk to your doctor immediately. The following side effects may be seen with Dorzolamide/Timolol:
Very Common (affects more than 1 user in 10):
Burning and stinging of the eyes, taste disturbances Common (affects 1 to 10 users in 100):
Redness in and around the eye(s), watering or itching of the eye(s), and effects on the surface of the eye(s), swelling and/or irritation in and around the eye(s), damage to the front layer of the eyeball (corneal erosion), decreased corneal sensitivity (not realising that something has gotten into the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or fullness in the nose), feeling sick, also called nausea, and tiredness (fatigue) Uncommon (affects 1 to 10 users in 1,000):
Dizziness, depression, inflammation of the iris, blurred vision (in some cases due to withdrawal of medication to treat excessive contraction of the pupil of the eye), slow heartbeat (bradycardia), fainting (syncope), indigestion (dyspepsia), and kidney stones (often marked by a sudden onset of excruciating, cramping pain in the lower back and/or side, groin, or abdomen).
Rare (affects 1 to 10 users in 10,000):
Systemic lupus erythematosus (an immune disease which may cause an inflammation of internal organs), tingling or numbness of hands and feet (Raynaud's phenomenon), unusual sensations (like pins and needles), difficulty sleeping (insomnia), nightmares, memory loss, weakening of the muscles, sexual dysfunction, decreased libido, stroke, temporary shortsightedness which may resolve when treatment is stopped, detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, drooping of the upper eyelid (making the eye stay half closed), double vision, eyelid crusting, swelling of the cornea (with symptoms of visual disturbances), low pressure in the eye, ringing noises in your ear, low blood pressure, irregular heartbeat, chest pain, palpitations (a quicker and/or irregular heartbeat), heart attack, reduced blood supply to the brain, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), reduced circulation in your arms and legs, leg cramps and/or leg pain when walking (claudication), shortness of breath, feeling out of breath, runny or stuffed nose, nose bleed, difficulty breathing, cough, throat irritation, dry mouth, diarrhoea, contact dermatitis, hair loss, psoriasis or worsening of psoriasis, Peyronie's disease (which may cause a curvature of the penis), muscle weakness/tiredness, allergic type reactions such as skin rash, hives, itchiness, in rare cases possible swelling of the lips, eyes and mouth, wheezing.
Not known (frequency cannot be estimated from the available data):
Low blood glucose levels, oedema (fluid build up), severe sudden life-threatening allergic reaction, increases in signs and symptoms of myasthenia gravis (muscle disorder), constriction of the airways in the lungs (predominantly in patients with pre-existing disease), muscle pain not caused by exercise, skin rash with white silvery coloured appearance (psoriasiform rash), heart failure, abdominal pain, vomiting.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist, particularly if you experience any changes/ visual disturbance when using Dorzolamide/Timolol after eye surgery.
5. HOWTO STORE DORZOLAMIDE/TIMOLOL
Keep out of the reach and sight of children.
Do not use Dorzolamide/Timolol after the expiry date which is stated on the bottle label and the carton after EXP:. The expiry date refers to the last day of that month.
This medicinal product does not require any special temperature storage conditions.
Dorzolamide/Timolol should be used within 28 days after the bottle is first opened. Therefore, you must throwaway the bottle 4 weeks after you first opened it, even if some solution is left. To help you remember, write down the date that you opened it in the space on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.These measures will help to protect the environment.
6. FURTHER INFORMATION
What Dorzolamide/Timolol contains
- The active substances are Dorzolamide and Timolol.
Each ml contains 20mg dorzolamide (as 22.26mg of dorzolamide hydrochloride) and 5mg timolol (as 6.83mg of timolol maleate).
- The other ingredients are Mannitol (E421), Hydroxy Ethyl cellulose, Benzalkonium chloride (as a preservative), Sodium citrate (E331), Sodium Hydroxide (E524)for pH adjustment and Water for injections.
What Dorzolamide/Timolol looks like and contents of the pack
Your medicine is in the form of a sterile, clear, slightly viscous, colourless aqueous eye drop solution.
Dorzolamide/Timolol is presented in a white opaque medium density polyethylene bottle with a sealed low density polyethylene dropper tip and a high density polyethylene cap with tamper proof seal, containing 5ml of the ophthalmic solution.
Pack sizes: 1,3 or 6 bottles of 5ml each.
Not all pack sizes may be marketed.
Marketing Authorisation Holder Pharmathen S.A.
6 Dervenakion str.
153 51 Pallini Attiki Greece
Manufacturer
Pharmathen S.A.
6 Dervenakion str.
153 51 Pallini Attiki
Greece
or
FamarS.A.
63 Agiou Dimitriou Street 17456 Alimos Greece
This leaflet was last approved in 01/2012 1010077/P2.8