Rizatriptan 10mg Orodispersible Tablets
PACKAGE LEAFLET: INFORMATION FOR THE USER
SZ00000LT000
Rizatriptan
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Rizatriptan is and what it is used for
2. Before you take Rizatriptan
3. How to take Rizatriptan
4. Possible side effects
5. How to store Rizatriptan
6. Further information
The active ingredient in this medicine, rizatriptan belongs to a group of medicines called 5-HT1B/1D receptor agonists. Your doctor has prescribed this medicine to treat the headache phase of your migraine attacks.
Do NOT take Rizatriptan if you:
• are allergic (hypersensitive) to rizatriptan or any of the other ingredients of Rizatriptan (see Section 6 and end of Section 2).
• are currently taking monoamine oxidase (MAO) inhibitors such as moclobemide, phenelzine, tranylcypromine (used to treat depression) or linezolid (drug used to treat bacterial infections), or have taken MAO inhibitors within the last two weeks (see section Taking other medicines’).
• have severely impaired liver or kidney function.
• have had a previous stroke (cerebrovascular accident or CVA) or symptoms similar to a stroke which wear off after a day or two (transient ischaemic attack or TIA).
• have moderately severe or severe high blood pressure, or mild high blood pressure that is NOT controlled by medication.
• have ever suffered from heart disease (poor blood flow in the arteries of the heart), heart attack or a particular type of chest pain known as Prinzmetal’s angina.
• have had problems with the blood supply to your legs (peripheral vascular disease).
• are taking any other medicine for your migraine such as ergotamine, ergotamine-type medicines (dihidroergotamine, methysergide), or another medicine in the same class (i.e. 5-HT1B/1D receptor agonists, such as sumatriptan, naratriptan or zolmitriptan)(see section Taking other medicines’).
Take special care with Rizatriptan
Before taking Rizatriptan, tell your doctor, if you have:
• any of the following risk factors for heart disease:
- high blood pressure or diabetes
- you smoke or are using a nicotine substitute
- a family history of heart disease
- you are a man over 40 years, or a post-menopausal woman.
• kidney or liver problems .
• a particular problem with the way your heart beats (bundle branch block).
• ever had any allergies.
• a headache associated with dizziness, difficulty in walking, lack of coordination or weakness in the leg and arm.
• had an allergic reaction to these or similar tablets such as swelling of the face, lips, tongue and/or throat which may cause difficulty breathing and/or swallowing.
• had short-lived symptoms including chest pain and tightness.
If you take Rizatriptan too often you may get a chronic headache. If this happens, you should contact your doctor as you may have to stop taking these tablets.
Rizatriptan should only be used for patients over the age of 18 years.
If you are older than 65 years, your doctor will advise whether you can take these tablets.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
DO NOT take Rizatriptan with:
Monoamine oxidase (MAO) inhibitors
such as moclobemide, phenelzine, linezolid or tranylcypromine, or if it is less than two weeks since you stopped taking an MAO inhibitor.
Certain other migraine medicines i.e.
• other medicines in the same class as rizatriptan, for example sumatriptan, naratriptan or zolmitriptan.
• ergotamine-type medicines such as ergotamine, dihydroergotamine or methysergide. You should wait at least 6 hours before taking these medicines after taking Rizatriptan, and you should allow at least 24 hours between stopping ergotamine-type medicines and starting Rizatriptan.
Ask your doctor for instructions and the risks about taking Rizatriptan if you are also taking:
• antidepressants such as sertraline, escitalopram, fluoxetine, venlafaxine, and duloxetine.
• propranolol (usually used to treat high blood pressure)
- you should receive only the lower 5 mg dose of Rizatriptan.
• the herbal remedy St. John’s Wort (Hypericum perforatum). Taking this together with Rizatriptan may increase the likelihood of side effects. It is recommended that you do not take Rizatriptan at the same time.
Taking Rizatriptan with food
It is better to take these tablets on an empty stomach, but you can also take it after you have had a meal. If Rizatriptan is taken with food, it may take longer to work.
Pregnancy and breast-feeding
You must tell your doctor if you are pregnant or intend to become pregnant. If you are pregnant you may take Rizatriptan only if your doctor decides it is clearly needed.
If you are breast-feeding, or planning to breast-feed, consult your doctor before taking the medicine. You should avoid breast-feeding for 24 hours following treatment with Rizatriptan.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Migraine itself or treatment with Rizatriptan may cause sleepiness in some patients. Dizziness has also been reported in some patients receiving this medicine. If you experience these effects you should check your ability to drive or to operate machinery safely.
Important information about some of the ingredients of Rizatriptan
Aspartame is an ingredient in these tablets, which is a source of phenylalanine. It may be harmful for people with phenylketonuria (a metabolic disorder which prevents the normal breakdown of phenylalanine).
Please tell your doctor or pharmacist about your symptoms. Your doctor will decide if you have a migraine. You should only take Rizatriptan for a migraine attack.
Rizatriptan should not be used to treat headaches that might be caused by other, more serious conditions.
Always take Rizatriptan exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Rizatriptan is not intended for prevention of migraine. It will only work once an attack has started.
The usual dose for adults over 18 years
is 10 mg at the first signs of a migraine attack. However in certain patients the recommended dose is 5 mg. Your doctor will have decided which dose is appropriate for you and it is important you take your medicine as your doctor has instructed.
Most migraine attacks are relieved with one dose (one tablet) of Rizatriptan, but if your migraine is not relieved after a single tablet, DO NOT take a second tablet to treat the same migraine attack, but seek medical advice.
Even if one migraine is not relieved by Rizatriptan, it is still likely that your next migraine attack will respond to the medicine.
If you have ANOTHER migraine attack within 24 hours of the first attack, you can take one further tablet of Rizatriptan, but do not take more than two tablets in a 24 hour period. Always leave at least 2 hours between doses.
Method of administration
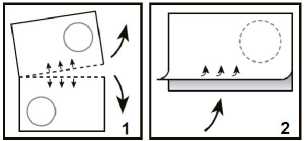
Take the orodispersible tablet as follows
1. Tear off one blister cell along the perforations.
2. Carefully peel off the backing, starting on the side indicated by the arrow.
Uncommon (affects 1 to 10 users in 1,000):
• poor muscle coordination, disorientation, sleeplessness, nervousness, spinning sensation
• blurred vision
• high blood pressure
• thirst, indigestion
• itching, itchy rash
• neck pain, feelings of tightness in parts of the body, stiffness, muscle weakness.
Rare (affects 1 to 10 users in 10,000):
• fainting, disturbed sense of taste (bad taste)
• wheezing
• facial pain.
Not known (frequency cannot be estimated from the available data):
• reduced blood supply to the hands and feet
• fits
• slower or irregular heart beat
• abnormal ECG heart tracing
• ischaemic colitis (inflammation which causes abdominal pain or diarrhoea)
• muscle pain.
As with other medicines in this class, there have been very rare reports of heart attack and stroke and most of these events have occurred in patients with risk factors for heart and blood vessel disease (high blood pressure, diabetes, smoking, family history of heart disease or stroke).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Rizatriptan after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Place the tablet on your tongue, where it will dissolve and be swallowed with the saliva.
You do not have to take a drink of water in order to swallow your tablet.
If you take more Rizatriptan than you should
It is important to keep to the dose the doctor has prescribed. If you do take more tablets than the doctor has advised, you should seek medical attention immediately as too many tablets can make you ill. The effects of taking too many tablets will include similar effects to those detailed in Section 4, especially: dizziness, sleepiness, fainting and slowed heart rate. You may also experience raised blood pressure and side effects affecting your heart and circulation.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Store in the original package.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Like all medicines, Rizatriptan can cause side effects, although not everybody gets them.
In clinical studies, the most frequently reported side effects were dizziness, sleepiness and tiredness.
If any of the following happen, contact your doctor immediately. These are rare serious side effects (affecting fewer than 1 in 1,000 people).
• allergic reactions sometimes very severe, including swelling of the face, lips, tongue and throat which may cause difficulty in breathing, speaking or swallowing
• severe allergic reaction with rash, red skin, blistering of the lips, eyes or mouth, skin peeling, and fever
• chest pain, tightness in the chest or throat or other symptoms consistent with heart attack
• weakness or paralysis of limbs or face, difficulty speaking which may indicate a stroke (see the end of this section)
• a syndrome called "serotonin syndrome" characterised by coma, unstable blood pressure, extremely high body temperature, lack of muscle coordination, agitation, and hallucinations.
Other possible side effects Common (affects 1 to 10 users in 100):
• headache, sensation of pins and needles, decreased sensitivity to touch and pain, decreased mental agility, trembling
• sensations of irregular and rapid heartbeat
• hot flushes/flashes
• throat discomfort, difficulty breathing
• nausea (feeling sick), dry mouth, vomiting, diarrhoea
• flushing of the skin, sweating
• feelings of heaviness in parts of the body
• stomach or chest pain.
What Rizatriptan contains
- The active substance is rizatriptan.
Rizatriptan 5 mg
Each orodispersible tablet contains 5 mg of rizatriptan (as rizatriptan benzoate).
Rizatriptan 10 mg
Each orodispersible tablet contains 10 mg of rizatriptan (as rizatriptan benzoate).
- The other ingredients are:
Calcium silicate; Crospovidone type A; Silica, Colloidal Anhydrous; Silicified Microcrystalline Cellulose; Mannitol (E421); Aspartame (E951); Magnesium Stearate; Sweet Orange Flavour (Arabic gum E414, Ascorbic acid E300, Ethyl butyrate, Maltodextrin, Orange oil).
What Rizatriptan looks like and contents of the pack
Orodispersible tablet Rizatriptan 5 mg: white to grey-white, round, flat tablet, debossed with “RZT” on one side, and “5” on the other side.
Rizatriptan 10 mg: white to grey-white, round, flat tablet, debossed with “RZT” on one side, and “10” on the other side.
Rizatriptan 5 mg Orodispersible Tablets: Al//Al blisters: 2, 3, 6, 18 orodispersible tablets.
Rizatriptan 10 mg Orodispersible Tablets: Al//Al blisters: 2, 3, 6, 12, 18 orodispersible tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder Sandoz Ltd,
Frimley Business Park, Frimley,
Camberley, Surrey, GU16 7SR, UK.
Manufacturer
Lek Pharmaceuticals d.d., Verovskova 57, 1526 Ljubljana, Slovenia or Lek Pharmaceuticals d.d., Trimlini 2D, 9220 Lendava, Slovenia or LEKS.A., ul.
Podlipie 16, 95-010 Strykow, Poland or LEK S.A., ul. Domaniewska 50 C, 02-672 Warszawa, Poland or Salutas Pharma GmbH, Otto-von-Guericke-Allee 1,39179 Barleben, Germany or Salutas Pharma GmbH, Dieselstrasse 5, 70839 Gerlingen, Germany or S.C. Sandoz S.R.L, Str. Livezeni nr. 7A, RO - 540472,
Targu-Mures, Romania.
This leaflet was last revised in 04/2013.
SZ00000LT000