Atrovent Udvs 2 Ml
Out of date information, search another|9ABJ} JO UO|lO0Jip
Package leaflet: Information for the user
Atrovent® UDVs®, 2 ml
(ipratropium bromide)
Read all of this leaflet carefully before you
start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets troublesome or serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What ATROVENT UDVs, 2 ml are and what they are used for
2. Before you use ATROVENT UDVs, 2ml
3. How to use ATROVENT UDVs, 2 ml
4. Possible side effects
5. How to store ATROVENT UDVs, 2 ml
6. Further information
1. WHAT ATROVENT UDVs, 2 ml ARE AND WHAT THEY ARE USED FOR
The name of your medicine is ATROVENT UDVs, 2 ml (called ATROVENT in the rest of this leaflet). You use it with a device called a ‘nebuliser’. This changes your medicine into a mist for you to breathe in.
ATROVENT contains a medicine called ipratropium bromide. This belongs to a group of medicines called bronchodilators. It is used to make breathing easier for people who have breathing difficulties, such as in chronic asthma or chronic obstructive pulmonary disease (COPD).
f\Boehringer lllr Ingelheim
ATROVENT can be taken at the same time as medicines called ‘beta2-agonist bronchodilators’ such as salbutamol.
ATROVENT works by opening up your airways.
2. BEFORE YOU USE ATROVENT UDVs, 2 ml
Do not use ATROVENT if:
• You are allergic (hypersensitive) to ipratropium bromide or any of the other ingredients in ATROVENT (listed in Section 6 below)
• You are allergic (hypersensitive) to medicines that are similar to ATROVENT, such as atropine
Do not use this medicine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using ATROVENT.
Take special care with ATROVENT
Check with your doctor or pharmacist before using your medicine if:
• You have cystic fibrosis
• You have glaucoma or have been told that you may develop it
• You are a man who has prostate problems
• You have problems passing water (urine)
• You are pregnant, likely to get pregnant or if you are breast-feeding
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using aTrOVENT.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription and herbal medicines. This is because ATROVENT can affect the way some other medicines work. Also some other medicines can affect the way ATROVENT works.
In particular, tell your doctor or pharmacist if you are taking any of the following:
• Medicines for breathing problems called ‘beta-agonists’ such as salbutamol
• Medicines for breathing problems called ‘xanthine preparations’ such as theophylline or aminophylline
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using aTrOVENT.
Pregnancy and breast-feeding
Talk to your doctor before using this medicine if you are pregnant, likely to get pregnant or are breast-feeding.
Driving and using machines
You may feel dizzy, or have difficulty in focusing, or blurred vision while taking ATROVENT. If this happens do not drive or use any tools or machines.
3. HOW TO USE ATROVENT UDVs, 2 ml
Always use ATROVENT exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. Follow these instructions to get the best results. If anything is unclear after reading this leaflet, ask your doctor or pharmacist.
How many dose units
Adults (including the elderly) and children
over 12 years
• The usual dose is the contents of k to 1 single dose unit (250-500 micrograms),
three to four times a day cj
• For acute attacks of breathlessness use ^ 1 single dose unit (500 micrograms)
• If breathlessness does not go away or gets worse consult your doctor
IT)
lO
Children under 12 years
• Not recommended for use
When children are using this medicine they must be supervised by a responsible adult.
Do not swallow or give this medicine by injection.
Do not use your nebuliser to take ATROVENT and ‘disodium cromoglycate inhalation solutions’ which have the preservative ‘benzalkonium chloride’ at the same time.
Do not use more than your doctor has told you
See your doctor straight away if:
• You feel that your medicine is not working as well as usual
• You need to use the nebuliser more than your doctor has recommended
Your doctor may need to check how well your medicine is working. In some cases your doctor may need to change your medicine.
|
File information |
Manda TD |
tory in Printfile | |||||
|
Issue date of TD: 06/JUN/2014 |
Yes |
Yes | |||||
|
PPM SKU: P012598 |
No |
Yes | |||||
|
PPM SKU version: 003 |
No |
Yes | |||||
|
Issue date of artwork: 06.06.2014 |
■ |
No |
Yes | ||||
|
Print colors: PAN Black |
No |
Yes | |||||
|
Mat. No. Pack. Site: 65NOT1011/C |
No |
Yes | |||||
|
Min. font size: 8.8pt | |||||||
|
Legend case version: V4.0 01/OCT/2012 (please do not change or remove it) | |||||||
MESSAGE TO OUR SUPPLIER :
Page 1 is always the front side. The text direction can be in landscape or portrait.
|
Technical information | |
|
a = Batch No. |
b = Expiry date |
|
c = Manufacturing date |
d = Price/Sample/Clinic |
Technical colors
BI-Diecut-Legendcase
Free area
Gluepoints
|
Additional information | ||
|
Bottle pack : |
4010/3-8-9-11 |
VjJNITHER |
|
Dimension : |
280 x 200 mm | |
|
Dimension of the leaflet after folding : |
35 x 200 mm | |
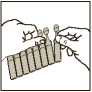
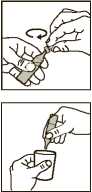
3. • Twist off the top
• Always hold it upright while you do this
6.
How to use your nebuliser
Read through numbers 1 to 6 first, before starting to use your nebuliser.
1. Get your nebuliser ready by following the manufacturer’s instructions. Ask your doctor if you are not sure how to use it.
2. • Open the pouch and remove the strip of unit dose vials. Carefully separate a new dose unit from the strip • Do not use it if it is already open
4. • Squeeze all the contents of the dose unit into the nebuliser chamber
• Your doctor will tell you if you need to use a different amount
• If you have also been prescribed a medicine called a ‘short-acting beta2-agonist nebuliser solution’ such as salbutamol the liquids can be mixed in the same nebuliser chamber
• If your doctor has told you that your medicine needs to be diluted, you will be given ‘sterile sodium chloride 0.9%’ solution. Your doctor will tell you how to do this
5. Use your nebuliser as directed by your doctor.
After you have finished, dispose of any
leftover medicine carefully
Follow the manufacturer’s instructions
on how to clean your nebuliser
It is important to keep your nebuliser
clean
Use a mouthpiece or a tight fitting mask. If any of the liquid or mist accidentally gets into your eyes you may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If you get problems with your eyes at any other time, talk to your doctor for advice.
If you use more ATROVENT than you should
If you use more of this medicine than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you.
If you forget to use ATROVENT
• If you forget a dose, use it as soon as you remember
• However, if it is nearly time for the next dose, skip the missed dose
• Do not use a double dose to make up for a forgotten dose
4. POSSIBLE SIDE EFFECTS
Like all medicines, ATROVENT can cause side effects, although not everybody gets them.
Stop using ATROVENT and see a doctor straight away, if you notice any of the following serious side effects - you may need urgent medical treatment:
• If after using ATROVENT you are wheezy or have other difficulties in breathing, do not use any more (unless you have been told to by your doctor).
• Allergic reactions - the signs may include skin rash and itching (affects less than 1 in 100 people). In severe cases the signs include swelling of your mouth and face, sudden difficulties in breathing and reduction of your blood pressure. Tightening of your throat (affects less than 1 in 100 people)
• Palpitations (fast or uneven heart beats) or quickening of the heart rate (affects less than 1 in 100 people)
• Increased heart rate or irregular heart rhythm such as atrial fibrillation (affects less than 1 in 1000 people)
Stop using this medicine and see your doctor straight away if you have any of these side effects.
Other side effects include:
Common (affects less than 1 in 10 people)
• Headache, dizziness
• Dry mouth, feeling sick (nausea), stomach upset or discomfort
• Cough and throat irritation when you have just used ATROVENT
Uncommon (affects less than 1 in 100 people)
• Itching, skin rash
• Unexpected tightness of the chest, swelling of the throat, dry throat
• Blurred vision, dilated pupils, glaucoma, painful, stinging, red or swelling of the eyes, see colours or lights
• Diarrhoea, constipation or being sick
• Mouth or lip sores
• Problems passing water (urine), especially if you already have problems passing urine
Rare (affects less than 1 in 1000 people)
• Difficulty focusing
• Nettle rash (urticaria)
If any of the liquid or mist accidentally gets into your eyes you may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If affected, do not drive or use any tools or machines. If you get problems with your eyes at any other time, talk to your doctor for advice.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www. mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ATROVENT UDVs, 2 ml
Do not use this medicine after the expiry date which is stated on the carton and the vial label. The expiry date refers to the last day of the month.
Store below 25°C.
Keep vials in the outer carton in order to protect from light.
Keep out of the sight and reach of children.
6. FURTHER INFORMATION What ATROVENT UDVs, 2 ml contain
ATROVENT UDVs, 2 ml contain a nebuliser solution. Each single dose unit contains 500 micrograms of the active ingredient ipratropium bromide as Ipratropium Bromide monohydrate Ph. Eur. in 2 ml of solution.
The other ingredients are: sodium chloride, hydrochloric acid and purified water.
What ATROVENT UDVs, 2 ml looks like and contents of the pack
Your medicine comes with a nebuliser. This changes ATROVENT into a mist for you to breathe in. ATROVENT UDVs, 2 ml are available in cartons containing 20 and 60 single dose units.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation for ATROVENT UDVs, 2 ml is held by:
Boehringer Ingelheim Limited,
Ellesfield Avenue, Bracknell, Berks.,
RG12 8YS, United Kingdom.
ATROVENT UDVs 2 ml, are manufactured by:
Laboratoire Unither
Espace Industriel Nord
151 rue Andre Durouchez - CS 28028
80084 Amiens Cedex 2, France
This leaflet was revised in June 2014.
© Boehringer Ingelheim Ltd 2014
65NOT1011/C