Azithromycin 250 Mg Film-Coated Tablets
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Azithromycin 250 mg Film-coated tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains azithromycin dihydrate equivalent to 250 mg of azithromycin. Excipient: Lactose monohydrate. Each tablet contains 5.40 mg lactose monohydrate.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Film-coated tablet.
White to off-white, capsule shaped, film-coated tablets of approximately 13.7 mm length, 6.8 mm width and 5.9 mm thickness, debossed with ‘AZ’ and ‘250’ on one side and plain on other side of the tablet.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Treatment of the following bacterial infections induced by micro-organisms susceptible to azithromycin (see sections 4.4 and 5.1):
- infections of the lower respiratory tract: acute exacerbation of chronic bronchitis (adequately diagnosed) and mild to moderate community-acquired pneumonia;
- infections of the upper respiratory tract: sinusitis and pharyngitis/tonsillitis;
- acute otitis media;
- infections of the skin and soft tissues of mild to moderate severity e.g. folliculitis, cellulites, erysipelas;
- uncomplicated Chlamydia trachomatis urethritis and cervicitis.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
4.2 Posology and method of administration
Posology
Children and adolescents with a body weight above 45 kg, adults and the elderly:
The total dosage of azithromycin is 1500 mg, staggered over three days (500 mg once daily). Alternatively, the dosage may be staggered over five days (500 mg as a single dose on the first day, and then 250 mg once daily).
In the case of uncomplicated Chlamydia trachomatis urethritis and cervicitis, the dosage is 1000 mg as a single oral dose.
Children and adolescents with a body weight below 45 kg:
Tablets are not indicated for these patients. Other pharmaceutical forms of azithromycin may be used, such as suspensions.
Elderly patients:
The same dosage as in adult patients is used in the elderly. Since elderly patients can be patients with ongoing proarrhythmic conditions a particular caution is recommended due to the risk of developing cardiac arrhythmia and torsades de pointes. (see Section 4.4 Special warnings and precautions for use).
Patients with renal impairment:
Dose adjustment is not required in patients with mild to moderate renal impairment (GFR 10-80 ml/min) (see section 4.4).
Patients with hepatic impairment:
Dose adjustment is not required for patients with mild to moderate hepatic dysfunction (see section 4.4).
Method of administration
This medicine should be taken in a single daily dose. The tablets should be swallowed whole and may be taken with or without food. The length of treatment for various infectious diseases is set out above.
4.3 Contraindications
The use of azithromycin is contraindicated in patients with hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotic, or to any excipient listed in Section 6.1
4.4 Special warnings and precautions for use
The selection of azithromycin to treat an individual patient should take into account the appropriateness of using a macrolide antibacterial agent based on adequate diagnosis to ascertain the bacterial etiology of the infection in the approved indications and the prevalence of resistance to azithromycin or other macrolides.
In areas with a high incidence of erythromycin A resistance, it is especially important to take into consideration the evolution of the pattern of susceptibility to azithromycin and other antibiotics.
As for other macrolides, high resistance rates of Streptococcus pneumoniae have been reported for azithromycin in some European countries (see section 5.1). This should be taken into account when treating infections caused by Streptococcus pneumoniae.
In bacterial pharyngitis the use of azithromycin is recommended only in cases where first line therapy with beta-lactams is not possible.
Allergic reactions:
As with erythromycin and other macrolides, rare serious allergic reactions, including angioedema and anaphylaxis (rarely fatal), have been reported. Some of these reactions with azithromycin have resulted in recurrent symptoms and required a longer period of observation and treatment.
Renal failure:
No dose adjustment is necessary in patients with mild to moderate renal impairment (GFR 10-80 ml/min). In patients with severe renal impairment (GFR <10 ml/min) a 33% increase in systemic exposure to azithromycin was observed (see Section 5.2 Pharmacokinetic properties).
Hepatic failure:
Since liver is the principal route of elimination for azithromycin, the use of azithromycin should be undertaken with caution in patients with significant hepatic disease. Cases of fulminant hepatitis potentially leading to life-threatening liver failure have been reported with azithromycin (see Section 4.8). Some patients may have had pre-existing hepatic disease or may have been taken other hepatotoxic medicinal products.
In case of signs and symptoms of liver dysfunction, such as rapid developing asthenia associated with jaundice, dark urine, bleeding tendency or hepatic encephalopathy, liver function tests/ investigations should be performed immediately. Azithromycin administration should be stopped if liver dysfunction has emerged.
Ergot alkaloids and Azithromycin:
In patients receiving ergot derivatives, ergotism has been precipitated by coadministration of some macrolide antibiotics. There are no data concerning the possibility of an interaction between ergot and azithromycin. However, because of the theoretical possibility of ergotism, azithromycin and ergot derivatives should not be coadministered. (see section 4.5).
QT prolongation:
Prolonged cardiac repolarisation and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen in treatment with other macrolides including azithromycin (See section 4.8 Undesirable effects). Therefore as the following situations may lead to an increased risk for ventricular arrhythmias (including torsade de pointes) which can lead to cardiac arrest, azithromycin should be used with caution in patients with ongoing proarrhythmic conditions (especially women and elderly patients) such as patients:
- With congenital or documented QT prolongation.
- Currently receiving treatment with other active substances known to prolong QT interval such as antiarrhythmics of classes IA (quinidine and procainamide) and III (dofetilide, amiodarone and sotalol), cisapride and terfenadine; antipsychotic agents such as pimozide; antidepressants such as citalopram; and fluoroquinolones such as moxifloxacin and levofloxacin.
- With electrolyte disturbance, particularly in cases of hypokalaemia and hypomagnesemia.
- With clinically relevant bradycardia, cardiac arrhythmia or severe cardiac insufficiency.
Pneumococcal infections:
As for other macrolides, high resistance rates of Streptococcus pneumoniae (> 30 %) have been reported for azithromycin in some European countries (see section 5.1). This should be taken into account when treating infections caused by Streptococcus pneumoniae.
Due to cross-resistance existing among macrolides, in areas with a high incidence of erythromycin resistance, it is especially important to take into consideration the evolution of the pattern of susceptibility to azithromycin and other antibiotics (see section 5.1).
Superinfections:
Attention should be paid to possible symptoms of superinfections caused by nonsensitive causal agents such as fungi. A superinfection may require an interruption of the azithromycin treatment and initiation of adequate measures.
Neurological or psychiatric diseases:
Azithromycin should be administered with caution to patients suffering from neurological or psychiatric diseases.
Pseudomembranous colitis:
After the use of macrolide antibiotics pseudomembranous colitis has been reported. This diagnosis should therefore be considered for patients who suffer from diarrhoea after start of the treatment with azithromycin. Should pseudomembranous colitis be induced by azithromycin, then anti-peristaltics should be contraindicated.
Clostridium difficile associated diarrhea:
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including azithromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
Long term use:
There is no experience regarding the safety and efficacy of long term use of azithromycin for the mentioned indications. In case of rapid recurrent infections, treatment with another antibiotic should be considered.
Azithromycin is not indicated for the treatment of infected burn wounds.
In case of sexually transmitted diseases a concomitant infection by T. pallidum should be excluded.
Exacerbations of the symptoms of myasthenia gravis and new onset of myasthenia syndrome have been reported in patients receiving azithromycin therapy (see section 4.8).
This medicine is not suitable for treatment of severe infections where a high concentration of the antibiotic in the blood is rapidly needed.
Safety and efficacy for the prevention or treatment of MAC (Mycobacterium Avium Complex) in children have not been established.
Azithromycin Jubilant contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Antacids:
In a pharmacokinetic study investigating the effects of simultaneous administration of antacid with azithromycin, no effect on overall bioavailability was seen although peak serum concentrations were reduced by approximately 25%. In patients receiving both azithromycin and antacids, the drugs should not be taken simultaneously.
Cetirizine:
In healthy volunteers, coadministration of a 5-day regimen of azithromycin with cetirizine 20 mg at steady-state resulted in no pharmacokinetic interaction and no significant changes in the QT interval.
Didanosine (Dideoxyinosine):
Coadministration of 1200 mg/day azithromycin with 400 mg/day didanosine in 6 HIV-positive subjects did not appear to affect the steady-state pharmacokinetics of didanosine as compared with placebo.
Digoxin (P-gp substrates):
Concomitant administration of macrolide antibiotics, including azithromycin, with P-glycoprotein substrates such as digoxin, has been reported to result in increased serum levels of the P-glycoprotein substrate. Therefore, if azithromycin and P-gp substrates such as digoxin are administered concomitantly, the possibility of elevated serum concentrations of the substrate should be considered.
Zidovudine:
Single 1000 mg doses and multiple 1200 mg or 600 mg doses of azithromycin had little effect on the plasma pharmacokinetics or urinary excretion of zidovudine or its glucuronide metabolite. However, administration of azithromycin increased the concentrations of phosphorylated zidovudine, the clinically active metabolite, in peripheral blood mononuclear cells. The clinical significance of this finding is unclear, but it may be of benefit to patients.
Azithromycin does not interact significantly with the hepatic cytochrome P450 system. It is not believed to undergo the pharmacokinetic drug interactions as seen with erythromycin and other macrolides. Hepatic cytochrome P450 induction or inactivation via cytochrome-metabolite complex does not occur with azithromycin.
Ergotamine:
Due to the theoretical possibility of ergotism, the concurrent use of azithromycin with ergot derivatives is not recommended (see Section 4.4 Special warnings and special precautions for use).
Pharmacokinetic studies have been conducted between azithromycin and the following drugs known to undergo significant cytochrome P450 mediated metabolism.
Atorvastatin:
Coadministration of atorvastatin (10 mg daily) and azithromycin (500 mg daily) did not alter the plasma concentrations of atorvastatin (based on a HMG CoA-reductase inhibition assay).
However, post-marketing cases of rhabdomyolysis in patients receiving azithromycin with statins have been reported.
Carbamazepine:
In a pharmacokinetic interaction study in healthy volunteers, no significant effect was observed on the plasma levels of carbamazepine or its active metabolite in patients receiving concomitant azithromycin.
Cimetidine:
In a pharmacokinetic study investigating the effects of a single dose of cimetidine, given 2 hours before azithromycin, on the pharmacokinetics of azithromycin, no alteration of azithromycin pharmacokinetics was seen.
Coumarin-Type Oral Anticoagulants:
In a pharmacokinetic interaction study, azithromycin did not alter the anticoagulant effect of a single 15-mg dose of warfarin administered to healthy volunteers. There have been reports received in the post-marketing period of potentiated anticoagulation subsequent to coadministration of azithromycin and coumarin-type oral anticoagulants. Although a causal relationship has not been established, consideration should be given to the frequency of monitoring prothrombin time when azithromycin is used in patients receiving coumarin-type oral anticoagulants.
Cyclosporin:
In a pharmacokinetic study with healthy volunteers that were administered a 500 mg/day oral dose of azithromycin for 3 days and were then administered a single 10 mg/kg oral dose of cyclosporin, the resulting cyclosporin Cmax and AUC0-5 were found to be significantly elevated. Consequently, caution should be exercised before considering concurrent administration of these drugs. If coadministration of these drugs is necessary, cyclosporin levels should be monitored and the dose adjusted accordingly.
Efavirenz:
Coadministration of a 600 mg single dose of azithromycin and 400 mg efavirenz daily for 7 days did not result in any clinically significant pharmacokinetic interactions.
Fluconazole:
Coadministration of a single dose of 1200 mg azithromycin did not alter the pharmacokinetics of a single dose of 800 mg fluconazole. Total exposure and half-life of azithromycin were unchanged by the coadministration of fluconazole, however, a clinically insignificant decrease in Cmax (18%) of azithromycin was observed.
Indinavir:
Coadministration of a single dose of 1200 mg azithromycin had no statistically significant effect on the pharmacokinetics of indinavir administered as 800 mg three times daily for 5 days.
Methylprednisolone:
In a pharmacokinetic interaction study in healthy volunteers, azithromycin had no significant effect on the pharmacokinetics of methylprednisolone.
Midazolam:
In healthy volunteers, coadministration of azithromycin 500 mg/day for 3 days did not cause clinically significant changes in the pharmacokinetics and pharmacodynamics of a single 15 mg dose of midazolam.
Nelfinavir:
Coadministration of azithromycin (1200 mg) and nelfinavir at steady state (750 mg three times daily) resulted in increased azithromycin concentrations. No clinically significant adverse effects were observed and no dose adjustment is required.
Rifabutin:
Coadministration of azithromycin and rifabutin did not affect the serum concentrations of either drug.
Neutropenia was observed in subjects receiving concomitant treatment of azithromycin and rifabutin. Although neutropenia has been associated with the use of rifabutin, a causal relationship to combination with azithromycin has not been established (see Section 4.8 Undesirable effects).
Sildenafil:
In normal healthy male volunteers, there was no evidence of an effect of azithromycin (500mg daily for 3 days) on the AUC and Cmax, of sildenafil or its major circulating metabolite.
Terfenadine:
Pharmacokinetic studies have reported no evidence of an interaction between azithromycin and terfenadine. There have been rare cases reported where the possibility of such an interaction could not be entirely excluded; however there was no specific evidence that such an interaction had occurred.
Theophylline:
There is no evidence of a clinically significant pharmacokinetic interaction when azithromycin and theophylline are co-administered to healthy volunteers.
Triazolam:
In 14 healthy volunteers, coadministration of azithromycin 500 mg on Day 1 and 250 mg on Day 2 with 0.125 mg triazolam on Day 2 had no significant effect on any of the pharmacokinetic variables for triazolam compared to triazolam and placebo.
Trimethoprim/sulfamethoxazole:
Coadministration of trimethoprim/sulfamethoxazole DS (160 mg/800 mg) for 7 days with azithromycin 1200 mg on Day 7 had no significant effect on peak concentrations, total exposure or urinary excretion of either trimethoprim or sulfamethoxazole. Azithromycin serum concentrations were similar to those seen in other studies.
CYP3A4 substrates:
Even though azithromycin does not appear to inhibit the enzyme CYP3A4, caution is advised when combining the medicinal product with quinidine, cyclosporine, cisapride, astemizole, terfenadine, ergot alkaloids, pimozide or other medicinal products with a narrow therapeutic index predominantly metabolised by CYP3A4.
Cisapride:
Cisapride is metabolized in the liver by the enzyme CYP 3A4. Because macrolides inhibit this enzyme, concomitant administration of cisapride may cause the increase of QT interval prolongation, ventricular arrhythmias and torsade de pointes.
Astemizol and, Alfentanil:
No data are available on interactions with astemizol, and alfentanil. Caution should be exercised with concomitant use of these agents and azithromycin in view of the described potentation of its effect during concomitant use of the macrolide antibiotic erythromycin.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate and well controlled studies on the use of azithromycin by pregnant women. Limited human data do not suggest an increase in the risk of birth defects.
In reproduction toxicity studies in animals azithromycin was shown to pass the placenta, but no teratogenic effects were observed (see section 5.3).
The safety of azithromycin has not been confirmed with regard to the use of the active substance during pregnancy. Therefore azithromycin should only be used during pregnancy if the benefit outweighs the risk.
Breast-feeding
Azithromycin has been reported to be secreted into human breast milk, but there are no adequate and well-controlled clinical studies in nursing women that have characterized the pharmacokinetics of azithromycin excretion into human breast milk.
A risk to the suckling infant cannot be excluded. Azithromycin should not be used in the treatment of a lactating woman unless the potential benefits justify the potential risks to the infant.
Fertility
In fertility studies conducted in rat, reduced pregnancy rates were noted following administration of azithromycin. The relevance of this finding to humans is unknown.
4.7 Effects on ability to drive and use machines
There is no evidence to suggest that azithromycin may have an effect on a patient’s ability to drive or operate machinery.
4.8 Undesirable effects
The table below lists the adverse reactions identified through clinical trial experience and post-marketing surveillance by system organ class and frequency. Adverse reactions identified from post-marketing experience are included in italics. The frequency grouping is defined using the following convention: Very common (>1/10); Common (> 1/100 to <1/10); Uncommon (>1/1,000 to <1/100); Rare (> 1/10,000 to <1/1,000); Very Rare (< 1/10,000); and Not known (cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Adverse reactions possibly or probably related to azithromycin based on clinical trial experience and post-marketing surveillance:
|
very common > 1/10 |
common > 1/100 to < 1/10 |
uncommon > 1/1,000 to < 1/100 |
rare > 1/10,000 to <1/1,000 |
very rare < 1/10,000 |
not known frequency cannot be estimated from available data |
|
Infections and infestations | |||||
|
Candidiasis Oral candidiasis Vaginal infection Pneumonia Fungal infection Bacterial infection Pharyngitis Gastroenteritis Respiratory disorder Rhinitis |
Pseudomembranous colitis (see 4.4) | ||||
|
Blood and lymphatic system disorders | |||||
|
Leukopenia Neutropenia Eosinophillia |
Thrombocytopenia, Haemolytic anaemia | ||||
|
Immune system disorders | |||||
|
Angioedema Hypersensitivity |
Anaphylactic reaction (see section 4.4.) | ||||
|
Metabolism anc |
nutrition disorders | ||||
|
anorexia | |||||
|
Psychiat |
ric disorders | ||||
|
Nervousness Insomnia |
Agitation |
Aggression Anxiety Dilirium Hallucination | |||
|
Nervous system disorders | |||||
|
Headache |
Dizziness Somnolence Paraesthesia Dysgeusia |
Syncope, Convulsion, Hypoaesthesia Psychomotor hyperactivity, Anosmia, Ageusia, Parosmia, Exacerbation or aggravation of myasthenia gravis (see 4.4) | |||
|
Eye disorders | |||||
|
Visual impairment | |||||
|
Ear and labyrinth disorders | |||||
|
Vertigo Ear disorder |
Hearing impairment including deafness and/or tinnitus | ||||
|
Cardiac disorders | |||||
|
palpitations |
Torsades de pointes (see Section 4.4) Arrhythmia (see Section 4.4) including ventricular tachycardia. Electrocardiogram QT prolonged (see Section 4.4) | ||||
|
Vascular disorders | |||||
|
Hot flush |
Hypotension | ||||
|
Respiratory, thoracic and mediastinal disorders | |||||
|
Dyspneu Epistaxis | |||||
|
Gastrointestinal disorders | |||||
|
Diarrhoea |
Vomiting Abdominal pain, Nausea |
Constipation Flatulence Dyspepsia Gastritits Dysphagia Abdominal distension Dry mouth Eructation Mouth ulceration Salivary hypersecretion |
Pancreatitis, Tongue discoloration | ||
|
Hepatobi |
iary disorders | ||||
|
Hepatitis |
Hepatic function abnormal Jaundice cholestatic |
Hepatic failure (see section 4.4), which has rarely resulted in death, Hepatitis fulminant | |||
|
Skin and subcutaneous tissue disorders | |||||
|
Rash, Pruritis Urticaria Dermatitis Dry skin Hyperhidrosis |
Photosensitivity reaction |
Stevens-Johnson syndrome, Toxic epidermal necrolysis, Erythema multiforme | |||
|
Musculoskeletal and connective tissue disorders | |||||
|
Osteoarthritis Myalgia Back pain Neck pain |
Arthralgia | ||||
|
Renal and urinary disorders | |||||
|
Dysuria Renal pain |
Renal failure acute, Nephritis interstitial | ||||
|
Reproductive system and breast disorders | |||||
|
Metrorrhagia Testicular disorder | |||||
|
General disorders and administration site conditions | |||||
|
Oedema Asthenia Malaise Fatigue Face edema Chest pain Pyrexia Pain Peripheral edema | |||||
|
Investigations | |||||
|
Lymphocyte count decreased, eosinophil count increased, blood bicarbonate decreased Basophils increased Monocytes increased Neutrophils increased |
Aspartate aminotransferase increased, alanine aminotransferase increased, blood bilirubin increased, blood urea increased, blood creatinine increased, blood potassium abnormal Blood alkaline phosphatase increased Chloride increased Glucose increased Platelets increased | ||||
|
Hematocrit decreased Bicarbonate increased abnormal sodium | |||||
|
Injury and poisoning | |||||
|
Post procedural complication | |||||
Adverse reactions possibly or probably related to Mycobacterium Avium Complex prophylaxis and treatment based on clinical trial experience and postmarketing surveillance. These adverse reactions differ from those reported with immediate release or the prolonged release formulations, either in kind or in frequency:
|
very common > 1/10 |
common > 1/100 to < 1/10 |
uncommon > 1/1,000 to < 1/100 |
|
Metabolism and nutrition disorders | ||
|
anorexia | ||
|
Nervous system disorders | ||
|
Dizziness Headache Paraesthesia Dysgeusia |
Hypoesthesia | |
|
Eye disorders | ||
|
Visual impairment | ||
|
Ear and labyrinth disorders | ||
|
Deafness |
Hearing impaired Tinnitus | |
|
Cardiac disorders | ||
|
palpitations | ||
|
Gastrointestinal disorders | ||
|
Diarrhoea Abdominal pain Nausea Flatulence Abdominal discomfort Loose stools | ||
|
Hepatobiliary disorders | ||
|
Hepatitis | ||
|
Skin and subcutaneous tissue disorders | ||
|
Rash, Pruritis |
Stevens-Johnson syndrome Photosensitivity reaction | |
|
Muscu |
oskeletal and connective tissue disorders | |
|
Arthralgia | ||
|
General t |
isorders and administration site conditions | |
|
Fatigue |
Asthenia | |
|
Malaise |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme: www.mhra.gov.uk/yellowcard
4.9 Overdose
Adverse events experienced in higher than recommended doses were similar to those seen at normal doses. In the event of overdosage, general symptomatic and supportive measures are indicated as required.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antibacterials for systemic use, macrolides ATC code: J01FA10
Azithromycin is a macrolide antibiotic belonging to the azalide group. The molecule is constructed by adding a nitrogen atom to the lactone ring of erythromycin A.
Mechanism of action:
The mechanism of action of azithromycin is based upon the suppression of bacterial protein synthesis, by binding to the ribosomal 50S sub-unit and thus inhibiting the translocation of peptides.
PK/PD relationship:
For azithromycin the AUC/MIC is the major PK/PD parameter correlating best with the efficacy of azithromycin.
Mechanism of resistance:
Generally, the resistance of different bacterial species to macrolides has been reported to occur by three mechanisms associated with target site alteration, antibiotic modification, or altered antibiotic transport (efflux). The efflux in streptococci is conferred by the mef genes and results in a macrolide-restricted resistance (M phenotype). Target modification is controlled by erm encoded methylases.
A complete cross resistance exists among erythromycin, azithromycin, other macrolides and lincosamides for Streptococcus pneumoniae, beta-haemolytic streptococcus of group A, Enterococcus spp. and Staphylococcus aureus, including methicillin resistant Staphylococcus aureus (MRSA).
Penicillin susceptible Streptococcus pneumoniae are more likely to be susceptible to azithromycin than are penicillin resistant strains of Streptococcus pneumoniae. Methicillin resistant Staphylococcus aureus (MRSA) is less likely to be susceptible to
The induction of significant resistance in both in vitro and in vivo models is <1 dilution rise in MICs for Streptococcus pyogenes, Haemophilus influenzae, and Enterobacterciae after nine sub lethal passages of active substance and three dilution increase for Staphylococcus aureus and development of in vitro resistance due to mutation is rare.
Breakpoints
Azithromycin susceptibility breakpoints for typical bacterial pathogens:
EUCAST:
- Staphylococcus spp.: susceptible < 1 mg/l; resistant > 2 mg/l
- Haemophilus spp.: susceptible < 0.12 mg/l; resistant > 4 mg/l
- Streptococcus pneumoniae and Streptococcus A, B, C, G: susceptible < 0.25 mg/l; resistant > 0.5 mg/l
- Moraxella catarrhalis: < 0.5 mg/l; resistant > 0.5 mg/l
- Neisseria gonorrhoeae: < 0.25 mg/l; resistant > 0.5 mg/l
The prevalence of resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. This information provides only an approximate guidance on the probability of an organism being susceptible to azithromycin.
Table : Antibacterial spectrum of azithromycin
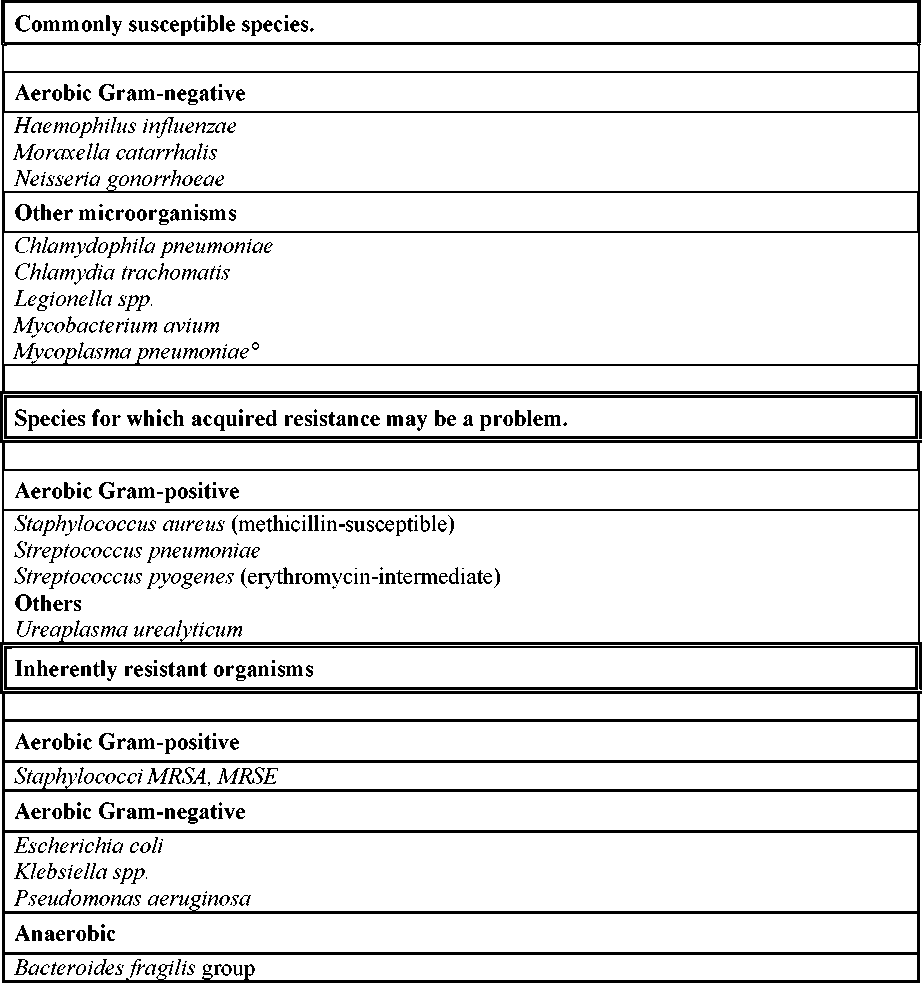
° At the time of publication there are no current data. In primary literature, standard works
and treatment guidelines susceptibility is assumed.
5.2 Pharmacokinetic properties
Absorption:
Following oral administration, the bioavailability of azithromycin is approximately 37 %. Peak plasma levels are reached after 2-3 hours. The mean maximum concentration observed (Cmax) after a single dose of 500 mg is approximately 0.4 pg/ml.
Distribution:
Orally administered azithromycin is widely distributed over the whole body.
Pharmacokinetic studies have shown considerably higher azithromycin concentrations in the tissues (up to 50 times the maximum concentration observed in the plasma) than in the plasma. This indicates that the substance is extensively bound in the tissues (steady-state volume of distribution approximately 31 l/kg).
With the recommended dosage no accumulation in the serum/plasma occurs. Accumulation does occur in the tissues where the levels are much higher than in the serum/plasma. Concentrations in target tissues such as lung, tonsil, and prostate exceed the MIC90 for likely pathogens after a single dose of 500 mg.
In experimental in-vitro and in-vivo studies, azithromycin accumulates in phagocytes; release is promoted by active phagocytosis. In animal models this process appeared to contribute to the accumulation of azithromycin in tissue. The binding of azithromycin to plasma proteins is variable, and varies from 52 % at 0.05 frg/ml to 18 % at 0.5 frg/ml, depending on the serum concentration.
Metabolism and Excretion:
The terminal plasma elimination half-life follows the tissue depletion half-life of 2 to 4 days.
Approximately 12 % of an intravenously administered dose is excreted in unchanged form with the urine over a period of 3 days; the major proportion in the first 24 hours. Concentrations of up to 237 frg/ml azithromycin, 2 days after a 5-day course of treatment, have been found in human bile.Ten metabolites have been identified (formed by N- and O-demethylation, by hydroxylation of the desosamine and aglycone rings, and by splitting of the cladinose conjugate). Investigations suggests that the metabolites do not play a role in the microbiological activity of azithromycin.
Pharmacokinetics in Special populations:
Renal Insufficiency:
Following a single oral dose of azithromycin 1 g, mean Cmaxand AUC0_i20 increased by 5.1 % and 4.2% respectively, in subjects with mild to moderate renal impairment (glomerular filtration rate of 10-80 ml/min) compared with normal renal function (GFR>80ml/min). In subjects with severe renal impairment, the mean Cmaxand AUC0-i20 increased 61% and 35% respectively compared to normal.
Hepatic insufficiency:
In patients with mild to moderate hepatic impairment, there is no evidence of a marked change in serum pharmacokinetics of azithromycin compared to normal hepatic function. In these patients, urinary recovery of azithromycin appears to increase perhaps to compensate for reduced hepatic clearance.
Elderly:
The pharmacokinetics of azithromycin in elderly men was similar to that of young adults; however, in elderly women, although higher peak concentrations (increased by 30-50%) were observed, no significant accumulation occurred.
In elderly volunteers (>65 years), higher (29 %) AUC values were always observed after a 5-day course than in younger volunteers (<45 years). However, these differences are not considered to be clinically relevant; no dose adjustment is therefore recommended.
Infants, toddlers, children and adolescents:
Pharmacokinetics has been studied in children aged 4 months - 15 years taking capsules, granules or suspension. At 10 mg/kg on day 1 followed by 5 mg/kg on days 2-5, the Cmax achieved is slightly lower than in adults, with 224 pg/l in children aged 0.6-5 years and after 3 days dosing, and 383 pg/l in those aged 6-15 years. The halflife of 36 h in the older children was within the expected range for adults.
5.3 Preclinical safety data
In animal studies using exposures 40 times those achieved at the clinical therapeutic dosages, azithromycin was found to have caused reversible phospholipidosis, but as a rule there were no associated toxicological consequences. The relevance of this finding to humans receiving azithromycin in accordance with the recommendations is unknown.
Electrophysiological investigations have shown that azithromycin prolongs the QT interval.
Carcinogenic potential:
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Mutagenic potential:
There was no evidence of a potential for genetic and chromosome mutations in in-vivo and in-vitro test models.
Reproductive toxicity:
Teratogenic effects were not observed in rat reproductive toxicity studies. At slight maternally toxic doses retardation in foetal ossification was seen. In peri- and postnatal studies in rats mild retardations in physical and reflex development were noted.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core:
Calcium hydrogen phosphate, anhydrous Hypromellose (E464)
Croscarmellose sodium Magnesium stearate (E470b)
Pregelatinised starch (maize) Sodium laurilsulfate
Tablet coat:
Hypromellose (E464) Lactose monohydrate Titanium dioxide (E171) Triacetin
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
White opaque, PVC/PVdC-Al blister.
Blister: 3, 4 or 6 tablets
Not all pack sizes may be marketed.
6.6
Special precautions for disposal
No special requirements.
7
MARKETING AUTHORISATION HOLDER
Jubilant Pharmaceuticals nv Axxes Business Park Guldensporenpark 22 - Block C 9820 Merelbeke Belgium
8
MARKETING AUTHORISATION NUMBER(S)
PL 19156/0056
9
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16/11/2010
10
DATE OF REVISION OF THE TEXT
03/12/2013