Betoptic 0.25% W/V Eye Drops Suspension
64953-0
I
I I
I
I
Package Leaflet - Information for the User
BETOPTIC* 0.25% w/v eye drops, suspension Betaxolol (as hydrochloride)
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others.
It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
1. What BETOPTIC 0.25 % is and what it is used for
2. Before you use BETOPTIC 0.25%
3. How to use BETOPTIC 0.25%
4. Possible side effects
5. How to store BETOPTIC 0.25%
6. Further information
l| WHAT BETOPTIC* 0.25% IS AND WHAT IT IS USED FOR~|
BETOPTIC 0.25 % belongs to a group of It is used to treat chronic open-angle
medicines known as beta blockers. glaucoma or ocular hypertension (high pressure
in the eye) by reducing the fluid pressure in your eye(s).
Do not use BETOPTIC 0.25% eye drops, suspension...
• If you are allergic to betaxolol, beta-blockers or any of the other ingredients listed in section 6.
• If you have now or have had in the past, respiratory problems such as severe asthma, severe chronic obstructive bronchitis (severe lung condition which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• If you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats).
Ask your doctor for advice.
Take special care...
Before you use this medicine, tell your doctor if you have now or have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure (hypotension)
• disturbances of heart rate such as slow heart beat (bradycardia)
• breathing problems, asthma or chronic obstructive pulmonary disease (lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• poor blood circulation disease (such as Raynaud’s disease or Raynaud’s syndrome)
• diabetes, as betaxolol may mask the signs and symptoms of low blood sugar
• overactivity of the thyroid gland as betaxolol may mask the signs and symptoms
• angle-closure glaucoma
• dry eyes (Sicca Syndrome)
Tell your doctor before you have an operation that you are using BETOPTIC 0.25% as betaxolol may change the effects of some medicines used during anaesthesia.
If any of these apply you may still be able to use BETOPTIC 0.25%, but discuss it with your doctor first.
Using other medicines
BETOPTIC 0.25 % can affect or be affected by other medicines you are using including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine, medicines to treat diabetes or medicines to treat emotional, behavioural or mental disorders such as anxiety or depression.
BETOPTIC 0.25% may reduce the effectiveness of adrenaline, which can be used to treat serious allergic reactions (anaphylaxis). Tell your doctor if you have a history of anaphylaxis or allergic reactions.
Please tell your doctor or pharmacist if
you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using more than one type of eye drop, wait 5 minutes between using each one. Eye ointments should be administered last.
Pregnancy and breast-feeding
Do not use BETOPTIC 0.25% if you are pregnant unless your doctor considers it necessary.
Do not use BETOPTIC 0.25% if you are breast-feeding. Betaxolol may get into your breast-milk.
Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
If your sight is affected in any way following the use of BETOPTIC 0.25%, you should not drive or use any machines.
Important information if you wear Contact Lenses
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in BETOPTIC 0.25% (benzalkonium chloride) that can discolour soft contact lenses.
BETOPTIC 0.25 % should only be used in the Remove the loose collar from the cap when
eye(s) you open the bottle.
Not recommended for use in CHILDREN
Always use BETOPTIC 0.25% exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
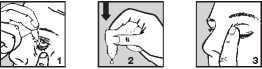
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
• Pull down your lower eyelid with a finger, until there is a ‘pocket’ between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid,
surrounding areas or other surfaces with the tip of the vial. It could infect the drops.
• Gently press on the base of the bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• After using BETOPTIC 0.25%, press a finger into the corner of your eye by the nose (picture 3) for 2 minutes. This helps to stop betaxolol getting into the rest of the body.
• If a drop misses your eye, try again.
• If you miss a dose, just take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not use a double dose to make up for a missed dose.
• If you use more BETOPTIC 0.25 % than you should it can be washed out of your eye with warm water.
If you have any further questions on the use
of BETOPTIC 0.25%, ask your doctor or pharmacist.
Like all medicines, BETOPTIC* 0.25% eye drops suspension can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you are worried, talk to a doctor or pharmacist. Do not stop using BETOPTIC 0.25% without speaking to your doctor.
Like other medicines applied into eyes, betaxolol is absorbed into the blood. This may cause similar side effects as seen with beta-blockers given by injection or taken by mouth. Incidence of side effects after beta-blockers are used in the treatment of eye conditions is lower than when medicines are, for example, taken by mouth or injected. Listed side effects include reactions seen following treatment with Betaxolol eye drops and within the class of other beta-blockers used for treating eye conditions.
Side effects experienced by patients during clinical trials with BETAXOLOL eye drops are:
Very common: may affect more than 1 in 10 users
• Eye discomfort (includes a feeling of something in the eye)
Common: (affects 1 to 10 users in 100)
• Blurred vision, watery eyes
• Headache
Uncommon (affects 1 to 10 users in 1000) :
• Inflammation of the eye surface, conjunctivitis or symptoms of conjunctivitis, visual impairment, sensitivity to light, painful, dry or tired eyes, excessive blinking, irritated, red or swollen eyes, a feeling of something in the eye, eye itchiness, eye discharge, weeping eyelids, bloodshot eyes.
• Slow heart beat or unusually rapid heart beat
• Asthma, difficulty breathing, blocked nose
• Nausea
Rare (affects 1 to 10 users in 10,000):
• Cataract formation, decreased sensitivity of the eye, inflammation of the eyelid
• Anxiety, difficulty sleeping (insomnia), depression
• Fainting
• Low blood pressure
• Cough, runny nose
• Taste disturbances
• Inflamed , itchy skin or rash, hair loss
• Libido decreased
The following side effects have also been reported by people using BETAXOLOL eye drops. The frequency cannot be estimated from the available data:
• Hypersensitivity reaction
• Dizziness
• Changes in the rhythm or speed of the heartbeat
• Loss or lack of strength
Additional side effects have been seen with other ophthalmic beta blockers and could potentially occur with BETOPTIC eye drops. The frequency is unknown.
• Generalised allergic reactions including swelling beneath the skin (that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing), hives
(or itchy rash), localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
• Low blood glucose levels.
• Nightmares, memory loss, hallucinations, delusions and confusion.
• Stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), unusual sensations (like pins and needles).
• Detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed), double vision.
• Chest pain, palpitations, oedema (fluid build up), congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
• Raynaud's phenomenon, cold hands and feet with a blue colour, leg pains (especially if you have a history of poor circulation).
• Constriction of the airways in the lungs (predominantly in patients with pre-existing disease ).
• Indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
• Skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis.
• Muscle pain not caused by exercise.
• Sexual dysfunction, impotence.
• Tiredness.
An increase in anti-nuclear antibodies has also been seen in patients taking ophthalmic beta-blockers
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
• Keep out of the reach and sight of children.
• Do not store above 25 °C.
• Store the bottle in the original package in order to protect from light.
• Keep the bottle tightly closed.
• Do not use the drops after the expiry date (marked ‘Exp') on the bottle and the carton. The expiry date refers to the last day of that month.
• Stop using the bottle 4 weeks after first opening, to prevent infections.
• Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• Do not pass this medicine on to others. It may harm them even if their symptoms are the same as yours.
What BETOPTIC 0.25% contains
• The active substance is betaxolol 2.5 mg/ml (as the hydrochloride).
• The other ingredients are disodium edetate, benzalkonium chloride, poly(styrene divinylbenzene) sulphonic acid, carbomer, boric acid, mannitol, N-lauroylsarcosine, hydrochloric acid and/or sodium hydroxide (to adjust pH) and purified water.
What BETOPTIC 0.25% looks like and contents of the pack
BETOPTIC 0.25% is a colourless, milky liquid supplied in a pack containing 5 ml plastic bottle with a screw cap.
Marketing authorisation holder:
Alcon Laboratories (UK) Ltd.
Frimley Business Park,
Frimley, Camberley,
Surrey, GU16 7SR,
United Kingdom.
Manufacturer:
SA Alcon-Couvreur NV,
Rijksweg 14, B-2870 Puurs, Belgium.
This leaflet was last revised in August 2014.
* a trademark ot Novartis © 2009, 201 1, 2012, 2013, 2014 Novartis
®
08-201 a Novartis company 64953-0