Bisoprolol Fumarate 10 Mg Film-Coated Tablets
Pharmacode position may change as per Supplier's m/c requirement & additional small pharma code may appear on the front / back panel
00
in'
O ■



Package leaflet: Information for the user
BISOPROLOL FUMARATE 5 mg film-coated tablets BISOPROLOL FUMARATE 10 mg film-coated tablets
Bisoprolol fumarate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
The name of your medicine is 'Bisoprolol fumarate 5mg film-coated tablets' or 'Bisoprolol fumarate 10mg film-coated tablets' but will be referred to as 'Bisoprolol fumarate'
1. What BISOPROLOL FUMARATE is and what they are used for
2. What you need to know before you take BISOPROLOL FUMARATE
3. How to take BISOPROLOL FUMARATE
4. Possible side effects
5. How to store BISOPROLOL FUMARATE
6. Contents of the pack and other information
1. What BISOPROLOL FUMARATE is and what it is used for
BISOPROLOL FUMARATE belongs to a group of medicines called beta-blockers. These medicines protect the heart against too much activity.
Bisoprolol may be used to treat angina pectoris (pains in the chest caused by blockages in the arteries that supply the heart muscle) or hypertension (high blood pressure).
2. What you need to know before you take BISOPROLOL FUMARATE
Do not take BISOPROLOL FUMARATE if you:
• are allergic (hypersensitive) to bisoprolol fumarate or any of the other ingredients of this medicine (listed in section 6).
• have or have had severe wheezing or severe asthma
• have a slow heart rate (less than 60 beats per minute). Ask your doctor if you are not sure.
• have very low blood pressure.
• have severe blood circulation problems (may cause your fingers and toes to tingle or turn pale or blue).
• have certain serious heart rhythm problems
• have heart failure, which has just occurred or which has recently become worse or requires hospital treatment
• have a condition in which there is an accumulation of excessive acid in the body known as metabolic acidosis. Your doctor will be able to advise you.
• have untreated phaeochromocytoma, a rare tumour of the adrenal gland
Tell your doctor if you are not sure about any of the above. Warnings and precautions
Talk to your doctor or pharmacist before taking BISOPROLOL FUMARATE if you:
• have liver or kidney problems.
• have diabetes. Bisoprolol Fumarate Tablets can hide the symptoms of low blood sugar.
• have or have had psoriasis (a recurrent skin disorder involving scaling and dry skin rash).
• Are being treated for hypersensitivity (allergic) reactions. Bisoprolol may make your condition worse or more difficult to treat.
• have been treated for a condition called 'phaeochromocytoma'
(a rare tumour of the adrenal gland),
• have a thyroid problem. The tablets can hide symptoms of an overactive thyroid.
• have asthma or chronic lung disease
• are fasting from solid food.
• have any irregularity of the electrical system of the heart
• suffer from Prinzmetal's angina which is a type of chest pain caused by spasm of the coronary arteries that supply the heart muscle.
• have any problems with the circulation to your hands and feet.
• consult a doctor, attend hospital or the dentist for surgery involving an anaesthetic, let them know what medicines you are taking.
Other medicines and BISOPROLOL FUMARATE:
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
It is particularly important to mention any of the following drugs as their action may be affected:
• Medicines used for controlling blood pressure or medicines used for the treatment of heart problems, such as: amlodipine, amiodarone, clonidine, diltiazem, disopyramide, digoxin, dobutamine, isoprenaline, methyldopa, moxonidine, phenytoin, nifedipine, quinidine, rilmenidine, verapamil and beta blocking agents.
• Medicines for the treatment of depression and mental disorders such as, tricyclic antidepressants, phenothiazines, monoamine oxidase inhibitors and barbiturates.
• Medicines used as anaesthetics during an operation
• Anti-inflammatory medicines known as NSAIDS (for instance diclofenac, indomethacin, ibuprofen, naproxen)
• Medicine used for diabetes.
• Medicines used for malaria e.g. mefloquine
• Medicines used for migraine e.g. ergotamine
• Medicines for asthma
• Medicines for a blocked nose
• Medicines for glaucoma (increased pressure in the eye)
• Medicines used to dilate (widen) the pupil of the eye.
• Medicines used to treat Alzheimer's disease
All these medicines may influence your blood pressure and/or heart function.
With insulin and other medicines used for diabetes, Bisoprolol may mask the symptoms of a low blood sugar.
BISOPROLOL FUMARATE with food and drink and alcohol
BISOPROLOL FUMARATE may be taken with or without food and should be swallowed whole with a drink of water.
The dizziness and light-headedness that may be caused by BISOPROLOL FUMARATE can be made worse if you drink alcohol. If this happens to you, you should avoid drinking alcohol.
Pregnancy and breast-feeding and fertility
BISOPROLOL FUMARATE may be harmful to the pregnancy and/or the unborn child. There is an increased possibility of premature birth, miscarriage, low blood sugar level and reduced heart rate of the child. The growth of the baby may also be affected. Therefore, Bisoprolol should not be taken during pregnancy.
It is not known if bisoprolol is excreted in the breast milk and therefore it is not recommended while breastfeeding.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
These tablets may make you feel tired, drowsy or dizzy. If you suffer from these side effects, do not operate vehicles and/or machines. Be aware of the possibility of these effects particularly at the beginning of the treatment, with changes in medication and with use in combination with alcohol.
3. How to take BISOPROLOL FUMARATE
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The tablets should be swallowed whole with water.
Your doctor will tell you your correct dosage and will usually start with the lowest dose (5mg). The maximum recommended dose of 20 mg. The tablets should be taken at about the same time each day.
Patients with kidney disease
Patients with severe kidney disease should not exceed 10mg of bisoprolol once daily. Please consult your doctor before starting to use this medicine.
Patients with liver disease
Patients with severe liver disease should not exceed 10mg of bisoprolol once daily. Please consult your doctor before starting to use this medicine.
Children under 12 years and adolescents
Not recommended as there is no experience with this medicine in children under 12 years and adolescents.
The tablet can be divided into equal doses.
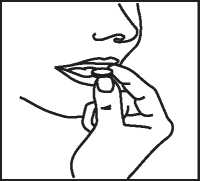
Take this tablet as follows:
1. Do not push the tablet
Do not push against the tablet pocket.
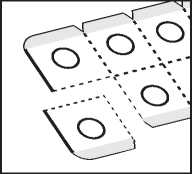
2. Tear off one tablet pocket
Bend and tear off one tablet pocket along the dotted lines.

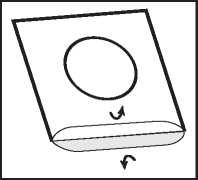
3. Peel off the lid
Lift, hold and peel off the lidding foil, starting from the side indicated by the arrow.
Dry eyes from reduced tear flow (can be very troublesome if you use contact lenses).
Inflammation of the liver (hepatitis), causing abdominal pain, loss of appetite and sometimes jaundice with yellowing of the whites of the eyes and skin, and dark urine.
Reduced sexual performance Fainting
Changes in blood test results
Very Rare: (may affect up to 1 in 10,000 people):
• aggravation of psoriasis or cause a similar dry, scaly rash
• Hair loss
• inflammation of the eye (conjunctivitis).
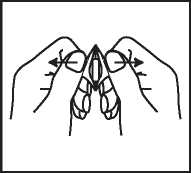
4. Remove the Tablet with dry hands and take as directed above.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store BISOPROLOL FUMARATE
If you take more BISOPROLOL FUMARATE than you should
If you have accidentally taken more than the prescribed dose, tell your doctor/pharmacist immediately. Take any remaining tablets or this leaflet with you so the medical staff know exactly what you have taken.
Symptoms of overdose may include dizziness, light-headedness, fatigue, breathlessness and/or wheezing. Also there may be reduced heart rate, reduced blood pressure, insufficient action of the heart and a low blood glucose level (which may involve feelings of hunger, sweating and palpitations).
If you forget to take BISOPROLOL FUMARATE
If you forgot to take a tablet, take it if you remember within 12 hours of your usual time. If more than 12 hours have passed, you should not take the missed tablet but should take your next tablet at the normal time when it is due. Do not take a double dose to make up for a forgotten tablet.
If you stop taking BISOPROLOL FUMARATE
Treatment with BISOPROLOL FUMARATE must not be stopped abruptly, particularly if you have had angina or a heart attack. If you suddenly stop the use of Bisoprolol fumarate your condition may get worse or your blood pressure may start to rise again. Instead, it must be reduced gradually over one or two weeks as advised by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Keep this medicine out of the sight and reach of children.
Store in the original package in order to protect from light.
Do not use this medicine after the expiry date which is stated on the carton/bottle after EXP.The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are important and will require immediate action if you experience them. You should stop taking Bisoprolol Fumarate Tablets and see your doctor immediately if the following symptoms occur:
Uncommon side effects (may affect up to 1 in 100 people):
• worsening of heart failure causing increased breathlessness and / or retention of fluid.
What BISOPROLOL FUMARATE contains
- The active substance is bisoprolol fumarate.
Each 5 mg film-coated tablet contains 5 mg of bisoprolol fumarate equivalent to 4.24 mg bisoprolol.
Each 10 mg film-coated tablet contains 10 mg of bisoprolol fumarate equivalent to 8.48 mg bisoprolol.
- The other ingredients are cellulose microcrystalline, calcium hydrogen phosphate anhydrous, silica colloidal anhydrous, crospovidone (Type A), magnesium stearate.
Tablet coat: hypromellose 6cP (E464), titanium dioxide (E171), macrogol 400, iron oxide yellow (E172).
What BISOPROLOL FUMARATE looks like and contents of the pack
Film-coated tablet.
BISOPROLOL FUMARATE 5 mg film-coated tablets
Yellow coloured, circular, biconvex, film-coated tablets debossed
with 'I and score line' on one side and '11' on the other side.
BISOPROLOL FUMARATE 10 mg film-coated tablets Yellow coloured, circular, biconvex, film-coated tablets debossed with 'I and score line' on one side and '13' on the other side.
BISOPROLOL FUMARATE film-coated tablets are available in -Polyamide/Aluminium/PVC/Paper/Polyester/Aluminium blisters and HDPE container with PP closure containing silica gel sachet.
Pack sizes:
Blister pack: 20, 28, 30, 50, 90, 100 film-coated tablets HDPE container pack: 30, 500 film-coated tablets
Not all pack sizes may be marketed.
Frequency not stated:
• worsening of symptoms of blockage of the main blood vessels to the legs, especially at the start of treatment.
The following side-effects have also been reported:
Common: (may affect up to 1 in 10 people):
• Tiredness, dizziness, headache (especially at the beginning of therapy; these symptoms are generally mild and often disappear within 1-2 weeks)
• cold or numbness of hands and/or feet
• Feeling sick (nausea), being sick (vomiting).
• Diarrhoea.
• Constipation.
• Low blood pressure
Uncommon: (may affect up to 1 in 100 people)
• Sleep disturbances.
• Depression.
• Slow or irregular heart beat.
• Patients with asthma or a history of breathing problems may experience difficulty in breathing.
• muscle weakness, cramps.
• feeling weak.
Rare: (may affect up to 1 in 1,000 people)
• Nightmares.
• Hallucinations.
• Hearing impairment.
• Inflammation of the lining of the nose, causing a runny nose with irritation.
• Allergic reactions (itching, flushed appearance, rash).
Marketing Authorisation Holder
Milpharm Limited
Ares, Odyssey Business Park
West End Road, South Ruislip HA4 6QD
United Kingdom
Manufacturer
APL Swift Services (Malta) Limited HF26, Hal Far Industrial Estate, Hal Far Birzebbugia, BBG 3000, Malta.
or
Vitama S.A. ul. Ceramiczna 1 05-850 Ottarzew Poland
This medicinal product is authorised in the Member States of the EEA under the following names:
France
Italy:
Poland:
Romania:
United Kingdom:
This leaflet was
Bisoprolol ARROW LAB 10 mg, comprime pellicule
Bisoprolol AUROBINDO 5 mg & 10 mg
compresse rivestite con film
Bisoprolol AUROBINDO
Bisoprolol Fumarat AUROBINDO 5 mg & 10 mg
comprimate filmate
BISOPROLOL FUMARATE 5 mg & 10 mg film-coated tablets
XXXXXSfd

last revised in 08/2016.