Boots Derma Care Eczema & Dermatitis Flare-Up 0.05% W/W Cream
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Diprolieve Eczema and Dermatitis Cream.
Boots Pharmacy Eczema and Dermatitis Cream
Boots Derma Care Eczema & Dermatitis Flare-Up 0.05% w/w Cream.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Alclometasone Dipropionate 0.05% w/w
For excipients, see 6.1
3 PHARMACEUTICAL FORM
Cream
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Diprolieve Eczema and Dermatitis Cream is indicated for the short-term treatment and control of patches of eczema and dermatitis including atopic eczema and primary irritant and allergic dermatitis.
4.2 Posology and method of administration
Route of administration - For cutaneous use.
Diprolieve Eczema and Dermatitis Cream is indicated for use in adults and children 12 years and older. Use in children under 12 years only on advice of a doctor.
Diprolieve Eczema and Dermatitis Cream should be applied sparingly (see section 6.6 Instructions for Use/Handling) to the affected area twice a day for up to 7 days. If the condition resolves within 7 days, treatment with Diprolieve Eczema and Dermatitis Cream should be stopped. If the condition does not improve in the first 7 days or becomes worse the consumer will be advised to see a doctor. If after 7 days of treatment improvement is seen but further treatment is required, the consumer will be
advised to see a doctor.
4.3 Contraindications
Hypersensitivity to Diprolieve Eczema and Dermatitis Cream or any of the ingredients; rosacea; acne and perioral dermatitis; tuberculous and viral lesions of the skin, particularly Herpes Simplex; vaccinia; varicella.
Diprolieve Eczema and Dermatitis Cream should not be used on broken skin or in fungal (eg candidiasis, tinea) or bacterial skin infections (eg impetigo).
4.4 Special warnings and precautions for use
As with all topical corticosteroids absorption can be increased by the use of occlusion, which in infants and children can lead to adrenal suppression. In addition, the management of eczema and dermatitis in infants and young children requires the supervision of a physician. Self-management is therefore limited to adults and children aged 12 years and over for no more than 7 days continuous treatment without occlusion.
Consumers will be advised not to initiate treatment of the same site for a third time without seeking medical advice to confirm the diagnosis.
Consumers should be advised to use Diprolieve Eczema and Dermatitis Cream only for the treatment of eczema or dermatitis, as it may mask or exacerbate other conditions. In particular, consumers will be advised not to use Diprolieve Eczema and Dermatitis Cream on the groin, breast-fold, genitals or between the toes as these are common sites of fungal infections.
Diprolieve Eczema and Dermatitis Cream should not be used on the face as it may cause acneform pustules or perioral dermatitis.
If irritation or sensitisation develops with the use of Diprolieve cream, treatment should be discontinued and appropriate therapy instituted.
Any of the side effects that have been reported following systemic use of corticosteroids, including adrenal suppression, may also occur with topical corticosteroids, especially in infants and children.
Systemic absorption of topical corticosteroids may be increased if extensive body surface areas are treated or if the occlusive technique is used. Suitable precautions should be taken under these conditions or when long-term use is anticipated, particularly in infants and children.
Paediatric Use: Paediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced hypothalamic-pituitary-adrenal (HPA) axis suppression and to exogenous corticosteroid effects than mature patients because of greater absorption due to a larger skin surface area to body weight ratio.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids.
Manifestations of adrenal suppression in children include low plasma cortisol levels, and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension including a bulging fontanelle, headaches, and bilateral papilledema.
Diprolieve Eczema and Dermatitis Cream is not for ophthalmic use.
Medical advice should be sought in seborrhoeic dermatitis since this involves areas of skin where Diprolieve Eczema and Dermatitis Cream should not be used.
Consumers should be warned not to use other topical corticosteroids, either prescribed or obtained over-the-counter (such as hydrocortisone) at the same time as Diprolieve Eczema and Dermatitis Cream as this may increase the risk of unwanted effects.
Consumers should be advised that they should not use topical alclometasone dipropionate for the treatment of psoriasis as rebound exacerbation may be a problem. This condition should be managed under the care of a physician.
4.5 Interaction with other medicinal products and other forms of interaction
None Known.
4.6 Fertility, pregnancy and lactation
Since safety of topical corticosteroid use in pregnant women has not been established, drugs of this class should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus. Drugs of this class should not be used extensively in large amounts for prolonged periods of time in pregnant patients.
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk.
Women who are pregnant or breast-feeding will be advised not to use Diprolieve Eczema and Dermatitis Cream but to seek the advice of a pharmacist or doctor.
4.7 Effects on ability to drive and use machines
There is no evidence that Diprolieve Eczema and Dermatitis Cream can have any effect on the ability to drive or operate machinery.
4.8
Undesirable effects
Adverse reactions reported rarely with aclometasone diproprionate are itching, burning, erythema,dryness, irritation, and popular rashes.
Other local adverse reactions associated with topical corticosteroids, especially under occlusive dressings, include folliculitis, hypertrichosis, acneform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, skin maceration, secondary infection, skin atrophy, striae and millaria.
Hypersensitivity and application site reactions, such as burning, stinging and irritation, have occurred rarely in a few patients following short-term (up to 7 days) use.
Consumers should be advised to stop treatment if signs of irritation and sensitisation occur.
Exacerbation of symptoms may occur.
4.9 Overdose
Acute overdosage with dermatologic application of corticosteroids is unlikely and would not be expected to lead to a life-threatening situation.
Symptoms: Excessive or prolonged use of topical corticosteroids can suppress
pituitary-adrenal function, resulting in secondary adrenal insufficiency, and produce manifestations of hypercorticism, including Cushing’s disease.
Treatment: Appropriate symptomatic treatment is indicated. Acute
hypercorticoid symptoms are usually reversible. Treat electrolyte imbalance, if necessary. In cases of chronic toxicity, slow withdrawal of corticosteroids is advised.
The steroid content is so low as to have little or no effect in the unlikely event of accidental oral ingestion.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Corticosteroids, dermatological preparations -Corticosteroids, moderately potent (group II), ATC code D07A B10
Alclometasone dipropionate is a non-fluorinated, topically active synthetic corticosteroid. Pharmacological studies in man and animals have demonstrated that alclometasone dipropionate suppresses local inflammation at doses producing minimal systemic effects. Preclinical studies have shown alclometasone dipropionate to be approximately 2/3 as potent as betamethasone valerate and 60 x as potent as hydrocortisone.
The anti-inflammatory properties of alclometasone dipropionate reduce the erythema, induration and pruritus associated with these conditions.
Diprolieve Eczema and Dermatitis Cream has no effect on hypothalamic-pituitary-adrenal function, as measured by plasma cortisol levels, as demonstrated by application of large amounts of the cream to healthy volunteer adults under whole body occlusion.
5.2 Pharmacokinetic properties
Not applicable in view of topical action and application.
5.3 Preclinical safety data
Alclometasone dipropionate cream appears to be a relatively non-toxic and non-irritating drug product that produces no unusual or unexpected teratologic effects in laboratory animals. A wide margin of safety was demonstrated in all species studied. Acute oral and intraperitoneal doses more than 3,000 times the proposed topical human dose were without any toxicologically significant effects.
Topical administration of corticosteroids to pregnant animals can cause abnormalities in foetal development. The relevance of this finding to human beings has not been established; however, topical steroids should not be used extensively in pregnancy i.e. in large amounts or for long periods.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Propylene glycol
White soft Paraffin
Cetostearyl alcohol
Glyceryl Stearate PEG 100 stearate
Polyoxyethylene (20) cetyl ether
Sodium dihydrogen phosphate dihydrate
Chlorocresol
Phosphoric acid
Purified Water
6.2 Incompatibilities
None Known.
6.3 Shelf life
60 months
6.4 Special precautions for storage
Do not store above 25 °C
6.5 Nature and contents of container
Aluminium tubes with white LDPE caps.
Pack sizes: 15g
6.6 Special precautions for disposal
Knowing how much cream to use can be difficult. The fingertip unit method is one easy way. A fingertip unit is the amount of cream you can squeeze on to your fingertip from the tip to the first crease. Half a fingertip unit (see diagram) will cover a patch of skin the same size as the palm of your hand.
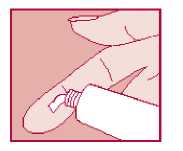
Follow these instructions:
• Wash your hands and dry them
• Squeeze out the correct amount of cream on to your index finger. The diagram gives you an idea of how much to use.
• Gently rub the cream in to the area of skin which you are treating, until the cream disappears
• Wash your hands again (unless it is your hands you are treating)
Use the fingertip unit as a guide. For smaller areas, use a smaller amount. The cream isn’t meant to treat large areas.
If you forget or miss a dose, use it when you remember.
Don’t worry if you use a bit too much cream by mistake, but try to keep to the fingertip unit. Using steroids on the skin continuously over many weeks or months can cause skin thinning.
Do not cover the treated area of skin with anything other than your clothes. Plasters, dressings, gloves or cling film should not be used as they can cause more of the medicine to pass through the skin.
7 MARKETING AUTHORISATION HOLDER
Merck Sharp & Dohme Limited
Hertford Road Hoddesdon Hertfordshire EN11 9BU UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 00025/0594
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
17/09/2009
10 DATE OF REVISION OF THE TEXT
05/03/2012