Brimonidine Tartrate 0.2% W/V Eye Drops
Package leaflet: Information for the user
BRIMONIDINE TARTRATE 0.2% w/v Eye drops
Brimonidine tartrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
• Your medicine, Brimonidine Tartrate 0.2% w/v Eye Drops will be referred to as Brimonidine in this leaflet. What is in this leaflet:
1. What Brimonidine is and what it is used for
2. What you need to know before you use Brimonidine
3. How to use Brimonidine
4. Possible side effects
5. How to store Brimonidine
6. Contents of the pack and other information
1. WHAT BRIMONIDINE IS AND WHAT IT IS USED FOR
Brimonidine Eye Drops are a sterile, preserved, aqueous solution used as eye drops. The active ingredient brimonidine tartrate works by reducing pressure within the eyeball. It is used to reduce pressure in the eye in the conditions of glaucoma or ocular hypertension. The medicine may be used alone or in conjunction with another eye drop that reduces pressure in the eye.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE BRIMONIDINE Do not use Brimonidine
• if you are allergic (hypersensitive) to Brimonidine or any of the other ingredients of this medicine (listed in section 6).
• if you are taking a monoamine oxidase (MAO) inhibitors medicine or a tricyclic antidepressants medicine (you should check with your doctor if you are taking any medicines for depression)
• if you are breast feeding
• in new born babies or children under 2 years Warnings and precautions
Talk to your doctor or pharmacist or nurse before using Brimonidine
• if you suffer from depression
• if you suffer from heart disease or heart problems
• if you suffer from dizziness or light-headedness on standing up (due to a fall in blood pressure)
• if you suffer from reduced mental capacity, reduced blood supply to the brain
• if you suffer from blood vessel disease in the limbs, or from poor blood circulation which makes the fingers or toes numb and pale
• if you have or have had in the past liver or kidney problems Children
• if it is intended for use in a child between the ages of 2 and 12, because it is not usually recommended for use in this age group
Other medicines and Brimonidine:
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
• a monoamine oxidase (MAO) inhibitors medicine or any other antidepressants
• sedative, barbiturate, opiates or hypnotic medicines to help you sleep or to sedate you or are regularly consuming alcohol.
• chlorpromazine, methylphenidate or reserpine (for mental/personality disorders)
• pain killers
• medicines for treating a heart condition or for lowering blood pressure
• medicines which work in the same way as brimonidine, eg. prazosin and isoprenaline
• if you are to be given anaesthetics, make sure you tell the doctor you are using these eye drops.
• medicines for any condition, even if unrelated to your eye condition
• or if the dose of any of your current medicine is changed Brimonidine with food and drink and alcohol
The effects of alcohol may be increased if you drink whilst using Brimonidine.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Brimonidine eye drops should not be used while breast-feeding.
Driving and using machines
• Brimonidine may cause blurred or abnormal vision after using Brimonidine. This effect may seem worse at night or in reduced lighting.
• Brimonidine may also cause drowsiness or fatigue in some patients.
• If you experience any of these symptoms, do not drive or use machinery until the symptoms are cleared. Brimonidine contains
The eye drops contain benzalkonium chloride as preservative which may cause eye irritation. Avoid contact with soft contact lenses. Remove contact lenses before using the eye drops and wait at least 15 minutes before reinserting. The preservative is known to discolour soft contact lenses.
3. HOW TO USE BRIMONIDINE
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is one drop into each affected eye twice daily, approximately 12 hours apart. If you are using in combination with another eye drop medicine, wait 5-15 minutes before applying the second eye drop. Use in Children
Brimonidine must not be used in infants under 2 years of age. It is not recommended for use in children between 2 to 12 years of age.
Instructions for use (Please also refer to pictograms at the end of the leaflet)
• first wash your hands
• avoid touching the eye (or any other surface) with the tip of the bottle
• if you wear soft contact lenses, they should be removed before using the eye drops and wait at least 15 minutes before reinserting
■ • these drops are supplied as a sealed bottle with a spiked cap. When using the bottle for the first time, screw the cap down tightly in order to pierce the tip of the bottle
• tilt your head back and look at the ceiling
• pull the lower eyelid gently downwards
• hold the bottle upside down above the eye and gently squeeze the bottle to release a drop into your eye
• keep the affected eye closed and press your fingertip against the inside corner of the closed eye, and hold for 1 minute
• repeat for the other eye if necessary
• replace and tighten the cap immediately after use.
Be careful not to touch the tip of the bottle on your eye or on any other surface.
Ocular solutions if handled wrongly can become contaminated by common bacteria and cause eye infections. If you do develop any other eye condition whilst using this product, see your doctor immediately.
If you use more Brimonidine than you should
In adults an overdose due to the use of the eye drops is unlikely. In children overdose has been reported in those
Page 1 of 2
receiving brimonidine as part of treatment for glaucoma. Contact your doctor immediately if a child develops signs of sleepiness, floppiness, low temperature or breathing difficulties. If you use too many drops or if the eye drops are accidentally swallowed, you should contact your doctor.

If you forget to use Brimonidine
Apply the drops as soon as you remember. However, if it is almost time for your next dose, do not double your dose and carry on with the normal schedule dose.
If you stop using Brimonidine
To be effective Brimonidine must be used every day. Do not stop using Brimonidine until your doctor tells you so.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience a rare (these may affect between 1 in 1,000 and 1 in 10,000 patients) but serious allergic reaction (difficulty breathing, closing of the throat, swelling of the lips, tongue, or face or hives) to brimonidine, stop using the medication and contact your doctor immediately.
Please tell your doctor if you notice any of the following side effects:
Very common: (these may affect more than 1 in 10 patients)
• an allergic reaction in the eye causing eye redness, burning, stinging, follicles or white spots on the membrane covering the inside of the eyelid/white of the eye, blurred vision, a feeling of something in the eye or itching
• headache, tiredness, drowsiness and dry mouth.
Common: (these may affect between 1 in 10 and 1 in 100 patients)
• changes to the surface of the eye, swollen or red eyelid, abnormal vision, sticky, weepy or watery eyes, sensitivity to light, irritation, pain, whitening of the membrane covering the inside of the eyelid/white of the eye
• inflammation of the see through layer which covers the surface of the eye
• dizziness, feeling or being sick, general weakness
• cold-like symptoms or abnormal taste.
Uncommon: (these may affect between 1 in 100 and 1 in 1,000 patients)
• depression
• palpitations or changes in heart rate
• dry nose or general allergic reactions
Rare: (may affect between 1 in 1,000 and 1 in 10,000 patients)
• shortness of breath
Very rare: (these may affect less than 1 in 10,000 patients)
• eye inflammation or a reduction in pupil size.
• fainting, high or low blood pressure or sleeplessness.
Some of the effects on the eye may be due to an allergy to the active ingredient or to any of the other ingredients. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE BRIMONIDINE
Keep all medicines out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after ’EXP’. The expiry date refers to the last day of that month.
Do not store above 25°C.
Discard after 28 days of opening.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Brimonidine contains
The active substance is brimonidine tartrate 0.2% w/v (2 mg/ml).
The other ingredients are 0.005% w/v benzalkonium chloride (as preservative), polyvinyl alcohol, sodium citrate, citric acid anhydrous, sodium chloride, sodium hydroxide and water for injection.
What Brimonidine looks like and contents of the pack
Each bottle contains 5 ml of the clear, greenish yellow coloured, eye drop solution.
Marketing Authorisation Holder and Manufacturer
FDC International Ltd, Unit 6, Fulcrum 1, Solent Way, Whiteley, Fareham, Hampshire, PO15 7FE
Tel: + 44 (0) 1489 565222
Email: fdcil@btconnect.com
PL number:15872/0018
This leaflet was last revised in 10/2014
MODE OF USE
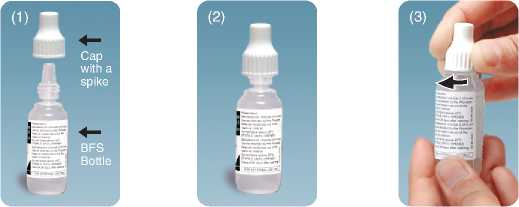
Bottle as Tighten the cap on the
received nozzle till the cap
touches the shoulder.

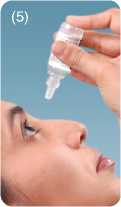
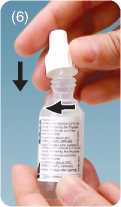
The spike in the cap Tilt head backwards. Dispense drops Replace cap after
will pierce the tip with gentle pressure. Do not touch every use, and screw
of the bottle. dropper tip to the surface of the eye. the cap down
_Page 2 of 2
Colour : CMYK Version : 04