Buprenorphine 8mg Sublingual Tablets
possible side effects not
used for
PACKAGE LEAFLET: INFORMATION FOR THE USER
Buprenorphine 2mg, 8mg Sublingual tablets
Buprenorphine Hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions please ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it onto others.
It may harm them even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any listed in this leaflet. See section 4.
What is in this leaflet:
1. What Buprenorphine 2mg and 8mg Sublingual tablets are and what they are
2. What you need to know before you take Buprenorphine 2mg and 8mg Sublingual tablets
3. How to take the tablets
4. Possible side effects
5. How to store the tablets
6. Contents of the pack and other information
1. What Buprenorphine 2mg and 8mg Sublingual tablets are and what they are used for
Buprenorphine 2mg and 8mg Sublingual tablets contain buprenorphine hydrochloride equivalent to 2mg or 8mg buprenorphine. Buprenorphine is one of a group of medicines used in opioid dependence. When it is used for the treatment of patients addicted to opiate (narcotic) drugs, such as morphine and heroin, it acts as a substitute for these drugs and therefore aids the patient in withdrawing from them over a period of time. If treatment is stopped abruptly, withdrawal symptoms can occur.
These tablets are described as 'sublingual'. This means that the tablet should be placed under the tongue and kept there until fully dissolved which usually occurs within 5 to 10 minutes.
2. What you need to know before you take Buprenorphine 2mg and 8mg Sublingual tablets
Do not take Buprenorphine 2mg and 8mg Sublingual tablets:
• If you are a child under the age of 16 years
• if you are allergic to buprenorphine or any of the other ingredients in this medicine (listed in section 6)
• if you have serious breathing problems
• i f you have serious problems with your liver or if your doctor detects the development of such a problem during treatment
• if you are intoxicated due to alcohol or have delirium tremens (the shakes and hallucinations)
• if you are breast-feeding Warnings and precautions
Tell your doctor if you have any of the following before treatment or develop them during treatment as your doctor may need to reduce your dose of this medicine or you may need extra treatment to control them:
• asthma or other breathing problems
• kidney disease
Some cases of severe liver problems have occurred during treatment although they may not necessarily have been caused by these tablets. If you develop severe fatigue, have no appetite or if your skin or eyes look yellow tell your doctor immediately.
This medicine can cause withdrawal symptoms if you take it less than four hours after you use a narcotic (morphine, heroin or other related products).
The tablets may cause your blood pressure to drop suddenly causing you to feel dizzy if you get up too quickly from sitting or lying down.
Drug dependence may occur as a result of taking this medicine.
Athletes should be aware that this medicine may cause a positive reaction to anti-doping tests.
As with other medicines of the opioid type, caution is needed for patients using buprenorphine, in the event of head injury, raised pressure within the head, low blood pressure, an enlarged prostate, or restriction of the urethra.
Other medicines and Buprenorphine 2mg and 8mg tablets?
You should not use benzodiazepines (medicines used to treat anxiety and sleep disorders) whilst you are taking this medicine unless they are prescribed by your doctor.
The tablets should be used exactly as prescribed by your doctor. Some people have died from respiratory failure (inability to breathe) whilst using benzodiazepines (medicines used to treat anxiety and sleep disorders) in combination with Buprenorphine and also when Buprenorphine was not used according to doctor's instructions.
Therefore whilst you are being treated with this medicine do not use benzodiazepines unless they have been prescribed by your doctor.
Strong pain killers and cough medicines containing opioid-related substances, certain anti-depressants including monoamine oxidase inhibitors, sedating antihistamines, sedatives, anti-anxiety drugs, certain drugs for high blood pressure, clonidine for migraine and antipsychotic drugs may increase the effects of buprenorphine.
Ketoconazole a medicine used for the treatment of fungal infections can increase the effects of Buprenorphine if both are taken at the same time. If you are taking ketoconazole or any of the following medicines: gestodene, troleandomycin, the HIV protease inhibitors ritonavir, indinavir and saquinavir you should tell your doctor or pharmacist as they may need to reduce your dose of buprenorphine.
Also tell your doctor or pharmacist if you are taking any of the following medicines: phenobarbital, carbamazapine, phenytoin, rifampicin. You will be closely monitored whilst you are taking these medicines at the same time as buprenorphine.
Be sure to tell your doctor if you are taking a blood thinning agent called phenprocoumon.
If you are taking any other medicines you should tell your doctor or pharmacist before you begin treatment with buprenorphine.
Buprenorphine tablets with alcohol
Do not drink alcohol or take medicines that contain alcohol whilst you are being treated with buprenorphine. Alcohol and certain other medicines (as listed above) increases the sedative effects of buprenorphine which can make driving or operating machinery hazardous.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or intend to become pregnant. When taken during pregnancy, particularly late pregnancy, medicines like Buprenorphine may cause drug withdrawal symptoms including problems with breathing in your new born baby. These symptoms may occur several days after birth. Do not breast feed your baby whilst taking this medicine as Buprenorphine passes into breast milk.
Driving and using machines
This medicine can cause drowsiness, which may be made worse if you also drink alcohol or take tranquillisers or anti-anxiety drugs. If you are drowsy do not drive or operate machinery.
This medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
° The medicine has been prescribed to treat a medical or dental problem and ° You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
° It was not affecting your ability to drive safely.
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Important information about some of the ingredients of Buprenorphine tablets
This product contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take Buprenorphine tablets
Always take this medicine as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Do not push the tablet out through the foil, this may damage the tablet.
Follow the diagram as indicated below.
2
2
o
co
<
o
hi
a.
i/> 2
a s
O) o «CO
(D < T3 U O uj u E
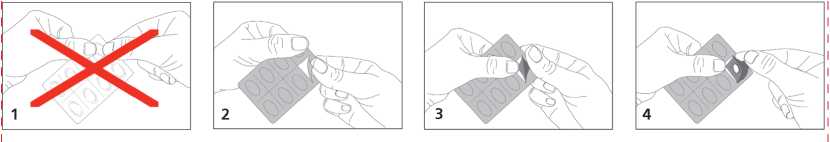
The tablets are administered sublingually. This means that you must place the tablet under your tongue and allow it to dissolve which will take 5 to 10 minutes. This is the only effective and safe way the tablets should be taken. Do not chew or swallow them whole as this will make them ineffective.
Your doctor will tell you how many 2mg or 8mg Sublingual tablets to take and you should always follow his advice.
The recommended dose is....
Adults and children over the age of 16 years: the initial dose is from 0.8 to 4mg administered once a day. For drug addicts who have not undergone withdrawal: one dose of 2mg or 8mg Sublingual tablets at least six hours after the last use of the opioid (narcotic) or when the first signs of craving appear.
For patients receiving methadone: before beginning treatment your doctor should reduce your dose of methadone to a maximum of 30mg a day.
These tablets may cause withdrawal symptoms in patients who are dependent on methadone.
During your treatment, your doctor may increase your dose to a maximum single daily dose of 16mg depending upon your response.
After a period of successful treatment, your doctor may gradually reduce your dose. Depending on your condition your dose may continue to be reduced under careful medical supervision until it is stopped altogether. Do not suddenly stop taking these tablets as this may cause withdrawal symptoms.
r — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — If you take more Buprenorphine than you should
You should contact your doctor immediately.
If you forget to take Buprenorphine
You should tell your doctor and follow his or her instructions. Do not take a double dose to make up for the forgotten dose.
If you stop taking Buprenorphine
Do not suddenly stop taking these tablets as this may cause withdrawal symptoms. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines Buprenorphine tablets can cause side effects, although not everybody gets them.
Tell your doctor immediately or seek urgent medical attention if you experience side effects such as :-
• sudden wheezing, difficulty breathing, swelling of the eyelids, face, tongue, lips, throat or hands; rash or itching especially those covering your whole body. These may be signs of a life threatening allergic reaction
• if you start to breathe more slowly or weakly than expected (respiratory depression)
• if you start to feel faint, as this may be a sign of low blood pressure.
Also tell your doctor immediately if you experience side effects such as:
• severe fatigue (tiredness), have no appetite or if your skin or eyes look yellow. These may be symptoms of liver damage.
The frequency of possible side effects listed below is defined using the following convention:
• common (affects 1 to 10 users in 100)
• Rare (affects 1 to 10 users in 10,000)
• not known (frequency cannot be estimated from the available data)
Side effects reported with Buprenorphine tablets
Common side effects:
Insomnia (inability to sleep), headache, fainting or dizziness (particularly when standing up), sweating, nausea (feeling sick), vomiting (being sick), constipation, drowsiness, feeling weak (lack of energy).
Rare side effects:
Hallucinations (seeing things that are not real), respiratory depression (breathing slowly or weakly), jaundice (yellowing of the eyes and skin), increased liver enzymes and liver swelling.
Frequency not known:
Hypersensitivity reactions such as skin rash, hives and itching. Breathing difficulties, wheezing, swelling of the eyes, lips, tongue, throat or hands.
Misusing this medicine by injecting it can cause withdrawal symptoms, infections, skin reactions, and potentially serious liver problems.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Buprenorphine tablets
Keep out of the sight and reach of children.
Do not use after the expiry date which is stamped on the container.
Medicines should not be disposed of via wastewater or household waste. Return any unused tablets to your pharmacist. These measures will help protect the environment.
6. Contents of the pack and other information
Each sublingual tablet contains buprenorphine hydrochloride equivalent to either 2mg or 8mg buprenorphine base as the active ingredient along with the following inactive ingredients: Lactose monohydrate, mannitol, maize starch, povidone K30, citric acid anhydrous, sodium citrate and magnesium stearate.
Buprenorphine tablets are white, uncoated oval tablets embossed with 2 or 8 on one side.
The sublingual tablets are available in cartons containing 7 tablets in a strip.
Marketing Authorisation Holder: Thornton & Ross Ltd., Linthwaite, Huddersfield, HD7 5QH, UK. Manufacturer: Fine Foods & Pharmaceuticals N.T.M. S.p.A, Via R. Follereau 25, 24027 Nembro (Bergamo), Italy. PL numbers: 00240/0354 2mg, 00240/0355 8mg This leaflet was last revised in February 2016
501261-01
24368803