Capd/Dpca 17 Solution For Peritoneal Dialysis
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
CAPD/DPCA 17
Solution for peritoneal dialysis
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 litre contains:
0.1838 g 5.786 g 7.85 g
0.1017 g 16.5 g
1.25 mmol/l 134 mmol/l 0.5 mmol/l 102.5 mmol/l 35 mmol/l 83.2 mmol/l
Calcium chloride diydrate Sodium chloride Sodium-(S)-lactate solution (3.925 g sodium-(S)-lactate)
Magnesium chloride hexahydrate Glucose monohydrate (15.0 g anhydrous glucose) up to 0.75 g fructose
Ca2+
Na+
Mg2+
Cl-
(S)-lactate
Glucose
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Solution for peritoneal dialysis
Clear colourless to slightly yellow solution
Theoretical osmolarity 356 mOsm/l pH » 5.5
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
End-stage (decompensated) chronic renal failure of any origin which can be treated with peritoneal dialysis.
4.2 Posology and method of administration
Posology
CAPD/DPCA 17 is exclusively indicated for intraperitoneal use.
The mode of therapy, frequency of administration, and dwell time required will be specified by the attending physician.
Continuous ambulatory peritoneal dialysis (CAPD)
Adults:
Unless otherwise prescribed, patients will receive an infusion of 2000 ml solution per exchange four times a day. After a dwell time between 2 and 10 hours the solution will be drained.
Adjustment of dosage, volume and number of exchanges will be necessary for individual patients.
If dilation pain occurs at the commencement of peritoneal dialysis treatment, the solution volume per exchange should be temporarily reduced to 500-1500 ml.
In large patients and if residual renal function is lost, an increased volume of dialysis solution will be necessary. In these patients, or patients who tolerate larger volumes, a dose of 2500 - 3000 ml solution per exchange may be given.
Children:
In children the solution volume per exchange should be prescribed according to age and body surface area (BSA).
For initial prescription, the volume per exchange should be 600-800 ml/m2 BSA with 4 (sometimes 3 or 5) exchanges per day. It can be increased up to 1000-1200 ml/m2 BSA depending on tolerance, age and residual renal function.
Automated peritoneal dialysis (APD)
If a machine (sleep safe cycler) is used for intermittent or continuous cyclic peritoneal dialysis, larger volume bags (e.g. 5000 ml) providing more than one solution exchanges are used. The cycler performs the solution exchanges according to the medical prescription stored in the cycler.
Adults:
Typically patients spend 8-10 hours a night cycling. Dwell volumes range from 1500 to 3000 ml and the number of cycles usually varies from 3 to 10 per night. The amount of fluid used is typically between 10 and 18 l but can range from 6 to 30 l. The cycler therapy at night is usually combined with 1 or 2 exchanges during the daytime.
Children:
The volume per exchange should be 800-1000 ml/m2 BSA with 5-10 exchanges overnight. It can be increased up to 1400 ml/m2 BSA depending on tolerance, age and residual renal function.
There are no special dosage recommendations for elderly patients.
Peritoneal dialysis is a long term therapy involving repeated administrations of single solutions.
Method and duration of administration
Patients must be trained appropriately, must practise the technique and be shown to be proficient at performing peritoneal dialysis before performing it at home. The training should be performed by qualified personnel. The attending physician must ensure that the patient masters the handling techniques sufficiently before the patient performs peritoneal dialysis at home. In case of any problems or uncertainty the attending physician should be contacted.
Dialysis using the prescribed doses should be performed daily and should be continued for as long as renal function substitution therapy is required.
Continuous ambulatory peritoneal dialysis (CAPD): staysafe bag The solution bag is first warmed to body temperature. For details see 6.6.
The appropriate dose is infused in the peritoneal cavity using a peritoneal catheter over 5 - 20 minutes. Depending on physician's instructions, the dose should dwell in the peritoneal cavity for 2 - 10 hours (equilibrium time), and then be drained.
Automated peritoneal dialysis (APD): sleewsafe bag
The connectors of the prescribed sleep safe solution bags are inserted in the free sleep safe tray ports and then automatically connected to the sleep safe tubing set by the cycler. The cycler checks the bar codes of the solution bags and gives an alarm when the bags do not comply with the prescription stored in the cycler. After this check the tubing set can be connected to the patient’s catheter extension and the treatment be started. The sleep safe solution is automatically warmed up to body temperature by the sleep safe cycler during the inflow into the abdominal cavity. Dwell times and selection of glucose concentrations are carried out according to the medical prescription stored in the cycler (for more details please refer to the operating instructions of the sleep safe cycler).
Depending on the required osmotic pressure, CAPD/DPCA 17 can be used sequentially with other peritoneal dialysis solutions with higher glucose content (i.e. with higher osmolarity).
4.3 Contraindications
For this specific peritoneal dialysis solution
CAPD/DPCA 17 must not be used in patients with lactic acidosis, severe hypokalaemia, and severe hypocalcaemia.
Due to the content of fructose, this medicinal product is not suitable for patients with fructose intolerance (hereditary fructose intolerance). A non-recognised hereditary fructose intolerance must be excluded prior to administration to babies and infants.
For peritoneal dialysis treatment in general
A peritoneal dialysis treatment should not be commenced in case of:
- recent abdominal surgery or injury, a history of abdominal operations with fibrous adhesions, severe abdominal burns, bowel perforation
- extensive inflammatory conditions of the abdominal skin (dermatitis),
- inflammatory bowel diseases (Crohn's disease, ulcerative colitis, diverticulitis),
- peritonitis,
- internal or external abdominal fistula,
- umbilical, inguinal or other abdominal hernia,
- intra-abdominal tumours,
- ileus,
- pulmonary disease (especially pneumonia),
- sepsis,
- extreme hyperlipidaemia,
- in rare cases of uraemia, which cannot be managed by peritoneal dialysis,
- cachexia and severe weight loss, particularly in cases in which the ingestion of adequate protein is not guaranteed,
- patients who are physically or mentally incapable of performing peritoneal dialysis as instructed by the physician.
If any of the above mentioned disorders develops during the peritoneal dialysis treatment, the attending physician has to decide on how to proceed.
4.4 Special warnings and precautions for use
The solution for peritoneal dialysis must not be used for intravenous infusion.
CAPD/DPCA 17 may only be administered after careful benefit-risk assessment in:
- loss of electrolytes due to vomiting and/or diarrhoea (a temporary change to a peritoneal dialysis solution containing potassium might then become necessary).
- hyperparathyroidism: The therapy should comprise the administration of calcium-containing phosphate binders and/or vitamin D to ensure adequate enteral calcium supply.
- hypocalcaemia: It may be necessary to use a peritoneal dialysis solutions with a higher calcium concentration either temporarily or permanently, in case an adequate enteral supply with calcium by calcium-containing phosphate binders and/or vitamin D is not possible.
- patients receiving digitalis therapy: Regular monitoring of the serum potassium level is mandatory. Severe hypokalaemia may necessitate the use of a potassium-containing dialysis solution together with dietary counselling.
Peritoneal dialysis solutions with a high glucose concentration (2.3 % or 4.25 %) should be used cautiously to protect the peritoneal membrane, to prevent dehydration and to reduce the glucose load.
A loss of proteins, amino acids, and water-soluble vitamins occurs during peritoneal dialysis. To avoid deficiencies an adequate diet or supplementation should be ensured.
The transport characteristics of the peritoneal membrane may change during longterm peritoneal dialysis primarily indicated by a loss of ultrafiltration. In severe cases peritoneal dialysis must be stopped and haemodialysis commenced.
Regular monitoring of the following parameters is recommended:
- body weight for the early recognition of over- and dehydration,
- serum sodium, potassium, calcium, magnesium, phosphate, acid base balance and blood proteins,
- serum creatinine and urea,
- blood sugar,
- parathormone and other indicators of bone metabolism,
- residual renal function in order to adapt the peritoneal dialysis treatment.
CAPD/DPCA 17 contains 15 g glucose in 1000 ml solution. Depending on the dosage instructions and the pack size used up to 45 g glucose (CAPD, 3000 ml stay safe) or 75 g glucose (APD, 5000 ml stay safe) are supplied to the body with each bag. This should be taken into account in patients with diabetes mellitus.
The effluent should be checked for clarity and volume. Turbidity and / or abdominal pain are indicators of peritonitis.
Elderly patients
The increased incidence of hernia should be considered in elderly patients prior to the start of peritoneal dialysis.
4.5 Interaction with other medicinal products and other forms of interaction
The use of this peritoneal dialysis solution can yield to a loss of efficacy of other medication if these are dialysable through the peritoneal membrane. A dose adjustment might become necessary.
A distinct reduction of the serum potassium level can increase the frequency of digitalis-associated adverse reactions. Potassium levels must be monitored particularly closely during concurrent digitalis therapy.
Special attention and monitoring is required in the case of hyperparathyroidism. Therapy should include the administration of calcium-containing phosphate binders and/or vitamin D to ensure adequate enteral calcium supply.
Use of diuretic agents may help maintain residual renal function, but may also result in water and electrolyte imbalances.
In diabetic patients the daily dose of insulin or oral hypoglycaemic medicinal products must be adjusted to take account of the increased glucose load.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data from the use of CAPD/DPCA 17 in pregnant women. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). CAPD/DPCA 17 should not be used during pregnancy unless the clinical condition of the woman requires treatment with CAPD 17 .
Breast-feeding
It is unknown whether CAPD/DPCA 17 active substances/metabolites are excreted in human milk.
Fertility
No data available.
4.7 Effects on ability to drive and use machines
CAPD/DPCA 17 has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Possible adverse reactions may result from the peritoneal dialysis treatment itself or may be induced by the dialysis solution.
The adverse drug reactions are ranked under the headings of reporting frequency, using the following convention:
|
very common |
>1/10 |
|
common |
>1/100 to <1/10 |
|
uncommon |
>1/1,000 to <1/100 |
|
rare |
>1/10,000 to <1/1,000 |
|
very rare |
<1/10,000 |
|
not known |
cannot be estimated from the available data |
Potential adverse reactions of the peritoneal dialysis solution Endocrine disorders
- Secondary hyperparathyroidism with potential disturbances of the bone metabolism (not known)
Metabolism and nutrition disorders
- Increased blood sugar levels (common)
- Increase in body weight due to the continuous uptake of glucose form the peritoneal dialysis solution (common)
- Hyperlipidaemia or deterioration of pre-existing hyperlipidaemia (common)
Cardiac and vascular disorders
- Hypotension (uncommon)
- Tachycardia (uncommon)
- Hypertension (uncommon)
Respiratory, thoracic and mediastinal disorders
- Dyspnoea (uncommon)
Renal and urinary disorders
- Electrolyte disturbances, e.g. hypokalaemia (very common)
- Hypocalcaemia (uncommon)
General disorders and administration site conditions
- Dizziness (uncommon)
- Oedema (uncommon)
- Disturbances in fluid balance (uncommon) indicated either by a rapid decrease
(dehydration) or increase (overhydration) in body weight. Severe dehydration might occur when using solutions of higher glucose concentration.
Potential adverse reactions of the treatment mode Infections and infestations
- Peritonitis (very common) indicated by a cloudy effluent. Later abdominal pain, fever, and general malaise may develop or, in very rare cases, sepsis. The patient should seek medical advice immediately.
The bag with the cloudy effluent should be closed with a sterile cap and assessed for microbiological contamination and white blood cell count.
- Skin exit site and tunnel infections (very common) indicated by redness, oedema, exudations, crusts and pain at the catheter exit site.
In case of skin exit site and tunnel infections the attending physician should be consulted as soon as possible.
Respiratory, thoracic and mediastinal disorders
- Dyspnoea caused by the elevated diaphragm (not known)
Gastrointestinal disorders
- Hernia (very common)
- Abdominal distension and sensation of fullness (common)
- Diarrhoea (uncommon)
- Constipation (uncommon)
Injury, poisoning and procedural complications
- In- and outflow disturbances of the dialysis solution (common)
- Shoulder pain (common)
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
No emergency situations in connection with overdose have been reported.
Any excess of dialysis solution infused in the peritoneal cavity can easily be drained into the drainage bag. In case of too frequent exchanges, dehydration and/or electrolyte disturbances can result which necessitate immediate medical attention. If an exchange has been forgotten, then the attending physician or dialysis centre in charge should be contacted.
Incorrect balancing can lead to hyper- or dehydration and electrolyte disturbances.
The most likely consequence of an overdosage with CAPD/DPCA 17 is dehydration. Underdosage, interruption of treatment or discontinuation of treatment may lead to life-threatening hyperhydration with peripheral oedema and cardiac decompensation and/or other symptoms of uraemia, which may endanger life.
The generally accepted rules for emergency care and intensive therapy must be applied. The patient may require immediate haemodialysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group Peritoneal dialytics, hypertonic solutions
ATC code B05D B
CAPD/DPCA 17 represents a lactate-buffered, glucose-containing electrolyte solution indicated for intraperitoneal administration for the treatment of end-stage renal failure of any origin by continuous ambulatory peritoneal dialysis (CAPD). The calcium dialysis concentration of this peritoneal dialysis solution is set at 1.25 mmol/l, which has been shown to reduce the risk of hypercalcaemia during the concomitant treatment with calcium-containing phosphate binders and/or vitamin D.
The characteristic of continuous ambulatory peritoneal dialysis (CAPD) is the more or less continuous presence of usually 2 litres of dialysis solution in the peritoneal cavity which is replaced by fresh solution three to five times a day.
The basic principle behind every peritoneal dialysis technique is the use of the peritoneum as a semipermeable membrane allowing the exchange of solutes and water between the blood and the dialysis solution by diffusion and convection according to their physico-chemical properties.
The electrolyte profile of the solution is basically the same as that of physiological serum, although it has been adapted (e.g. the potassium content) for use in uraemic patients to enable renal function substitution therapy by means of intraperitoneal substance and fluid exchange. Substances which are normally eliminated with the urine, such as urea, creatinine, inorganic phosphate, uric acid, other solutes and water, are removed from the body into the dialysis solution. It should be borne in mind that medication may also be eliminated during dialysis, and that a dose adjustment may thus be necessary.
Individual parameters (such as patient size, body weight, laboratory parameters, residual renal function, ultrafiltration) must be used to determine the dose and combination of solutions required with differing osmolarity (glucose content), potassium, sodium, and calcium concentrations. The efficacy of therapy should be regularly monitored on the basis of these parameters.
Peritoneal dialysis solutions with a high glucose concentration (2.3 % or 4.25 %) are used when the body weight is above the desired dry weight. The withdrawal of fluid from the body increases in relation to the glucose concentration of the peritoneal dialysis solution.
5.2 Pharmacokinetic properties
Uraemic retention products such as urea, creatinine, and uric acid, inorganic phosphate, and electrolytes such as sodium, potassium, calcium and magnesium are removed from the body into the dialysis solution by diffusion and/or convection.
Dialysate glucose used as an osmotic agent in CAPD/DPCA 17 is slowly absorbed decreasing the diffusion gradient between dialysis solution and extracellular fluid. Ultrafiltration is maximal at the beginning of the dwell time reaching a peak after about 2 to 3 hours. Later absorption starts with a progressive loss of ultrafiltrate. After 4 hours the ultrafiltrate averages 100 ml with a 1.5 %, 400 ml with a 2.3 %, and 800 ml with a 4.25 % glucose solution. 60 to 80 % of dialysate glucose are absorbed.
S-lactate used as the buffering agent is almost completely absorbed after a 6-hour dwell time. In patients with a normal hepatic function S-lactate is rapidly metabolised demonstrated by normal values of intermediate metabolites. Calcium mass transfer depends on the dialysis solution glucose concentration, the effluent volume, the serum ionised calcium, and the calcium concentration in the dialysis solution. The higher the glucose concentration, the effluent volume and the serum ionised calcium concentration, and the lower the calcium concentration in the dialysis solution, the higher is the calcium transfer from the patient to the dialysate. It has been estimated that a typical CAPD schedule of three 1.5% and one 4.25% glucose-containing bags per day would remove up to 160 mg calcium per day enabling a higher intake of oral calcium containing drugs and vitamin D without the risk of hypercalcaemia.
5.3 Preclinical safety data
No preclinical toxicity studies with CAPD/DPCA 17 have been carried out, but clinical studies with comparable solutions for peritoneal dialysis have shown no major risk of toxicity.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Hydrochloric acid 25% pH-correction
Sodium hydroxide pH-correction
Water for injections
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life 2 years
Shelf life after first opening: The content must be used immediately.
6.4 Special precautions for storage
Do not store above 25 °C. Do not refrigerate or freeze.
6.5 Nature and contents of container
stavsafe:
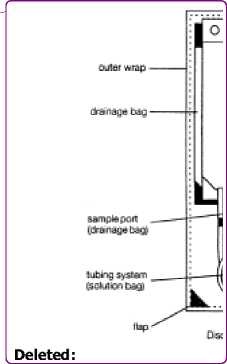
The stay safe system is provided as a double bag system consisting of a non-PVC solution bag made of a multi layer polyolefine based foil, a tubing system also made of polyolefines, a system connector (DISC, polypropylene), a drainage bag and an outer bag, also made of polyolefine multi layer film.
sleevsafe:
The sleep safe system is provided as a single bag system consisting of a non-PVC solution bag made of a multi layer polyolefine based foil, a tubing system, a bag connector both also made of polyolefines and an injection port made of polyolefine/synthetic rubber.
Pack sizes:
stay safe sleep safe
6 bags of 1500 ml each 2 bags of 5000 ml each
4 bags of 2000 ml each 4 bags of 2500 ml each 4 bags of 3000 ml each
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements for disposal.
stavsafe system:
The solution is first warmed to body temperature. For bags with a volume up to 3000 ml this should be done using an appropriate bag warmer. The heating time for a 2000 ml bag with a starting temperature of 22°C is approx. 120 min. The temperature control is done automatically and is set to 39°C ± 1°C. More detailed information can be obtained from the operating instructions of the bag warmer. Use of microwaves is not recommended due the risk of local overheating.
1. Check the solution bag (label, the expiry date and ensure that the solution is clear) - open the overwrap and the packaging of the disinfection cap.
2. Clean hands with an antimicrobial washing solution.
3. Place the DISC into the organiser (suspend solution bag from the upper hole of the infusion pole - unroll the line “solution bag-DISC” - place the DISC into the organiser - afterwards place drainage bag into lower holder of the infusion pole).
4. Place catheter extension into one of the two inserts of the organiser. Place the new disinfection cap into the other free insert.
5. Disinfect your hands and remove protection cap of the DISC
6. Connect catheter extension to the DISC:
7. Open clamp on extension - position “(” - outflow procedure starts.
8. After completion of the outflow: Flush-
position “((” -flush of fresh dialysate to the drainage bag (approx. 5 seconds)
9. Inflow - position “*)(” - connection
between solution bag and catheter.
10. Security step - position “((((” -automated closing of the catheter extension with the PIN.
11. Disconnection - remove the protection cap from the new disinfection cap and screw it onto the old one. Unscrew catheter extension from the DISC and screw onto the new disinfection cap.
12. Close the DISC with the open end of the protection cap (which has remained in the right hole of the organiser)
13. Check the drained dialysate for clarity and weight and, if the effluent is clear, discard it.
sleevsafe system (for the set up of the sleep safe system please refer to its operating instructions):
1. Preparation of the solution
• Check the solution bag (label, expiry date, clearness of the solution, bag, and overwrap not damaged).
• Place the bag on a solid surface.
• Open the overwrap of the bag.
• Wash your hands with an antimicrobial washing lotion.
• Check whether the solution is clear and that the bag is not leaking.
2. Unroll tubing of bag.
3. Remove the protection cap.
4. Insert connector in free sleep safe tray port.
5. The bag is now ready for use with the sleep safe set.
See also section 4.2.
Handling
Plastic containers may occasionally be damaged during transport or storage. This can result in a contamination with growth of microorganisms in the dialysis solution. Thus all containers should be carefully inspected for damage prior to connection of the bag and prior to use of the peritoneal dialysis solution. Any damage, even minor, to connectors, at the closure, container welds and corners, must be noted because of possible contamination.
Damaged bags or bags with cloudy content should never be used! In case of doubt the attending physician should decide on the use of the solution.
Only use the peritoneal dialysis solution if container and seal are undamaged.
The overwrap should only be removed before administration.
Aseptic conditions must be maintained during dialysate exchange in order to reduce the risk of infection.
Addition of medication to the peritoneal dialysis solution:
The addition of medication to the peritoneal dialysis solution is generally not recommended because of the risk of contamination and of incompatibility between the peritoneal dialysis solution and the medication.
When adding drugs, use aseptic technique, mix thoroughly and after checking for the absence of any turbidity, which may occur due to incompatibilities, the peritoneal dialysis solution must be used immediately.
7 MARKETING AUTHORISATION HOLDER
Fresenius Medical Care Deutschland GmbH
D-61346 Bad Homburg,
Germany
8 MARKETING AUTHORISATION NUMBER(S)
PL 13689/0001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
24/06/2009
10 DATE OF REVISION OF THE TEXT
17/02/2016