Ciclesonide 160 Micrograms/Dose Inhaler Cfc Free
Package Leaflet: Information for the User
Alvesco® 160 Inhaler
(ciclesonide)
This medicine is not suitable for use in an acute attack of breathlessness. For quick relief from such an attack, use only your Reliever inhaler.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
• The name of this medicine is Alvesco® 160 Inhaler but will be referred to as Alvesco throughout the remainder of this leaflet.
• This medicine is also available in other strengths.
What is in this leaflet:
1. What Alvesco is and what it is used for
2. What you need to know before you use Alvesco
3. How to use Alvesco
4. Possible side effects
5. How to store Alvesco
6. Contents of the pack and other information
1. What Alvesco is and what it is used for What Alvesco is:
Alvesco is a clear and colourless aerosol spray for you to breathe in through your mouth and into your lungs. It is a Preventer medication (corticosteroid) that has to be taken every day and which becomes active only after it has been inhaled into your lungs.
The active ingredient in this medicine is ciclesonide. (For the other ingredients, see section 6).
What Alvesco is used for:
This medicine is prescribed to control persistent asthma in adult and adolescent patients (12 years old and more).
It helps you to breathe more easily by decreasing the symptoms of your asthma and by lessening the chances of an asthma attack. The effect builds up over a period of time, so this medicine needs to be taken every day, even when you are feeling well.
2. What you need to know before you use Alvesco Do not use Alvesco
if you are allergic (hypersensitive) to ciclesonide or any of the other ingredients of this medicine (listed in section 6).
Take special care with Alvesco
• Before beginning treatment with this medicine, please tell your doctor if:
you have ever been treated, or are currently being treated, for pulmonary tuberculosis (TB), fungal, viral or bacterial infections.
Check with your doctor if you are not sure. It is important to make sure that Alvesco is the right medicine for you.
During your treatment with Alvesco contact your doctor immediately if:
• breathing becomes difficult and your symptoms, coughing, breathlessness, wheezing, tightness in the chest, increasing noises (rhonchi) or other symptoms of narrowing of the airways are getting worse.
(You should use your Reliever inhaler which will normally lead quickly to an improvement.)
• you are waking up at night with your symptoms.
• you are not getting relief from using your Reliever inhaler.
Your doctor will decide on your further treatment.
Specific patient groups
Patients with severe asthma are at risk of acute asthma attacks. For such patients the doctor will carry out regular thorough asthma control checks, including a lung function test.
Patients who are already taking corticosteroid tablets:
Alvesco can be used to replace your tablets, or to reduce the number of tablets you need to take. Please follow your doctor's instructions carefully.
• This will start about a week after you begin your Alvesco inhalations.
• The number of tablets you take will be reduced with caution over a period of time.
• During this period you may sometimes suffer from a general feeling of being unwell.
• In spite of this, it is important to continue both with your Alvesco inhalations and with slowly reducing the number of tablets you take.
• If you get serious symptoms such as nausea (feeling sick), vomiting (being sick), diarrhoea or a high temperature, contact your doctor.
• This process may sometimes reveal minor allergies such as rhinitis (inflammation of the inside of the nose) or eczema (itchy, reddening skin).
• If you have changed over from tablets, you will continue for a time to be at risk of reduced adrenal function, which is related to the corticosteroid tablets you take. The symptoms of reduced adrenal function (e.g. dizziness, fainting, nausea, loss of appetite, moodiness, decrease in body hair, inability to cope with stress, weakness, headaches, memory problems, allergies, food cravings, and blood sugar disorders) may also continue for some time.
• You may also need to see a specialist to determine the extent of the reduction in adrenal function.
• Your doctor will also do regular checks on your adrenal function.
• During periods of stress, for example, having an operation, worsening asthma attacks, it is possible that you will need extra corticosteroid tablets. If so, you must carry a steroid warning card which says so.
Patients with liver or kidney disorders:
There is no need to adjust the dose of ciclesonide if you have liver or kidney problems. If you suffer from a severe liver condition, your doctor will check you more carefully for possible side effects resulting from disturbance of normal steroid production.
Children below 12 years of age:
This medicine is not recommended for children below the age of 12 because of a lack of information about its possible effects.
Other medicines and Alvesco
Please inform your doctor before using Alvesco, if you are currently being treated for any fungal or viral infections with medicine containing:
• ketoconazole,
• itraconazole,
• ritonavir,
• nelfinavir.
These may intensify the action of Alvesco so that the probability of side effects cannot be completely ruled out.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using this medicine with food and drink
There is no interaction between Alvesco and food and drink.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant, want to become pregnant or are breast-feeding.
• Because there is not enough information about the effects of Alvesco on pregnant women, your doctor will discuss with you the risks and benefits of using Alvesco.
• Ciclesonide (the active ingredient in Alvesco) may be taken during pregnancy only when the possible benefits to the mother justify the possible risk to the developing baby. If your doctor decides that you can continue using Alvesco, the smallest possible dose of ciclesonide will be used to maintain asthma control.
• The adrenal function will be carefully monitored in children of mothers who received corticosteroids during pregnancy.
• Talk to your doctor if you want to use Alvesco during breast-feeding.
• It is not known whether inhaled ciclesonide passes into the breast milk in humans.
• Prescribing Alvesco to women who are breast-feeding will therefore only be considered if the expected benefit to the mother outweighs the possible risk to the child.
Ask your doctor or pharmacist for advice before taking any medicine. Driving and using machines
Alvesco and its ingredients have no or negligible effects on the ability to drive or to use machinery.
3. How to use Alvesco
Always use this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
• If you have just started to use this medicine instead of, or as well as, taking corticosteroid tablets, see section 2, Patients who are already taking corticosteroid tablets.
How much Alvesco should I take each day?
Your doctor will have spoken to you about how much of your medicine you need to take each day. This will depend on your individual need.
• The recommended dose of Alvesco is 160 micrograms once daily, which leads to asthma control in the majority of patients.
• In some patients a dose reduction to 80 micrograms, once daily, may be an adequate dose for maintaining effective control of their asthma.
• An increased dosage of Alvesco may become necessary for a short period of time in patients who suffer a severe worsening of their asthma symptoms. This can be up to 640 micrograms per day, delivered as 320 micrograms twice daily but no data confirming the additional therapeutic effect after 3 months with these higher doses are available.
If necessary, your doctor may also prescribe corticosteroid tablets and/or, in the case of an infection, an antibiotic.
• Your doctor will adjust your dose to the minimum necessary to control your asthma.
• You should start to notice an improvement in your symptoms (wheezing, tight chest and coughing) within 24 hours.
When should I use my Alvesco inhaler?
In most cases, either in the morning or in the evening - as one or two puffs once a day. Follow your doctor's instructions very carefully. It is important that you take Alvesco regularly every day, even if you feel better.
If you find that you have to use your Reliever inhaler more than 2-3 times a week, you should contact your doctor to have your medicine reviewed.
How do I use my Alvesco inhaler?
It is important that a doctor, nurse or pharmacist shows you first how to use your Alvesco inhaler properly. A good technique will make sure you are receiving the correct amount into your lungs. Please use the instructions in this leaflet as a reminder.
You may wish to practise in front of the mirror for the first couple of times until you are confident that you are using your Alvesco inhaler properly. Make sure that none of your medicine is escaping from the top or sides of your mouth.
If you have a new inhaler, or if you have not used your inhaler for a week or more, it must be tested before you use it. Remove the mouthpiece cover and press down three times on the canister inside the inhaler to release three puffs into the air - away from you.
You do not need to shake your Alvesco inhaler before using it. The medicine is already in a very fine solution, mixed to ensure you receive the correct dose with each puff.
1. Remove the mouthpiece cover and check the mouthpiece, both the inside and the outside, to make sure that it is both clean and dry.




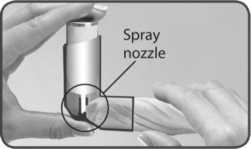
Follow these instructions carefully and use the pictures to guide you.
2. Hold the inhaler upside down (base of the canister at the top) with your forefinger on the base of the canister and your thumb under the mouthpiece.
3. Breathe out as far as is comfortable. Do not breathe out through the inhaler.
4. Place the mouthpiece in your mouth and close your lips firmly around it.
5. Just after starting to breathe in through your mouth, press down with your forefinger on the top of the inhaler to release a puff of the medicine while you are still breathing in slowly and deeply. Please take care that the puff of medicine does not escape through the top, bottom or sides of your mouth.
6. Hold your breath, take the inhaler from your mouth and remove your finger from the top of the inhaler. Continue holding your breath for about ten seconds or as long as is comfortable. Breathe out slowly through your mouth. Do not breathe out through the inhaler.
It is important that you do not rush
steps 3 to 6.
7. If you have been instructed to take another puff, wait about half a minute and repeat steps 3 to 6.
8. After use, always replace the mouthpiece cover to keep out dust. Replace firmly and snap into position.
9. For hygiene reasons
• please clean the mouthpiece weekly with a dry tissue, both inside and out.
• using a dry, folded tissue, wipe over the front of the small hole where the medicine comes out.
• do not use water or any other liquids.
A correct technique will ensure the right amount of Alvesco is getting into your lungs every time you use your inhaler. Your doctor will check your inhalation technique regularly to ensure that your treatment can have the very best effect.
When the canister is completely empty you will not feel or hear any of the propellant being discharged.
If you begin to feel wheezy or tightness in the chest after using your Alvesco inhaler:
• do not take any more puffs.
• use your reliever inhaler to help your breathing.
• contact your doctor immediately.
If you find it difficult to use the inhaler, your doctor may recommend the use of a spacer. The spacer that fits the Alvesco inhaler is called AeroChamber Plus™. If you use the AeroChamber Plus™ device, please follow the instructions provided with it. Your doctor or pharmacist will be able to advise you about the device.
If you use more Alvesco than you should
It is important that you take your dose as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
There is no specific treatment necessary if you have used too much Alvesco but you should inform your doctor. If high doses are used over long periods, a certain degree of reduction in adrenal function cannot be ruled out and control of the adrenal function may be necessary.
If you forget to use Alvesco
If you have forgotten to use your Alvesco, just take your usual dose when it is next due. Do not take a double number of puffs to make up for the forgotten dose.
If you stop using Alvesco
Even if you feel better, you should not stop using your Alvesco inhaler.
If you do stop using this medicine, you must tell your doctor immediately.
4. Possible side effects
Like all medicines, Alvesco can cause side effects, although not everybody gets them.
The other side effects seen with Alvesco are usually mild. In most cases you can continue with your treatment. The side effects you may experience are:
Uncommon side effects (may affect up to 1 in 100 patients treated):
• hoarseness
• burning, inflammation, irritation of mouth or throat
• oral thrush (oral fungal infection)
• headache
• bad taste
• dryness of mouth or throat
• nausea or vomiting
Rare side effects (may affect up to 1 in 1,000 patients treated):
• sensation of heartbeat (palpitations)
• discomfort or pain in the abdomen
• high blood pressure
Frequency not known, but may also occur:
• Sleeping problems, depression or feeling worried, restless, nervous, over-excited or irritable. These effects are more likely to occur in children
Alvesco may affect the normal production of corticosteroids in your body. This is usually seen in patients taking high doses over a long period of time. These effects may include:
• reduced rate of growth in adolescents
• a thinning of the bones
• possible clouding of the lens of the eye (cataracts) causing blurred vision
• loss of vision caused by abnormally high pressure in the eye (glaucoma)
• moon-shaped face, weight gain in the upper body and thinning arms and legs (Cushingoid features or Cushing syndrome).
Adolescents who are receiving treatment for a long period of time should have their height checked regularly by their doctor. If your growth rate is slowed, your doctor will adjust your treatment if possible to the lowest dose at which effective control of asthma is maintained.
Corticosteroid tablets can lead to more side effects than a corticosteroid inhaler such as Alvesco. If you have been taking steroid tablets before or during the use of Alvesco, the risk of side effects from the tablets may continue for a period of time. Regular check-ups with your doctor will ensure that you are taking the right dose of Alvesco for you. Regular check-ups will also identify any side effects early on and reduce the chances of them worsening.
Please remember:
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Alvesco
• Keep out of the sight and reach of children.
• Do not use your inhaler after the expiry date which is stated on the label and the carton after EXP. The expiry date refers to the last day of that month.
• The container contains a pressurised liquid. Do not expose to temperatures higher than 50°C.
• The container should not be punctured, broken or burned even if it seems empty.
• As with most inhaled medicines in pressurised containers, the healing effect of this medicinal product may become smaller when the container is cold. However, Alvesco delivers the same level of dose from minus 10°C to plus 40°C.
• If your medicine shows any sign of deterioration, return it to your pharmacist.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Alvesco contains
Each actuation releases a puff (dose delivered through the mouthpiece)
that contains 160 micrograms of the active ingredient ciclesonide.
The other ingredients are anhydrous ethanol and propellant HFA-134a
(norflurane).
What Alvesco looks like and contents of the pack
Alvesco is a clear and colourless liquid in a pressurised aluminium container fitted with a plastic metering valve, mouthpiece and a brown-reddish body and cap which delivers an accurately measured dose of ciclesonide in the form of a spray.
Alvesco comes in packs of 1 or 2 inhalers containing 60 metered actuations in each inhaler.
Manufactured by
Takeda GmbH, Byk-Gulden-Str. 2, D-78467 Konstanz, Germany
Procured from within the EU by the Product Licence Holder:
MPT Pharma Ltd, Westgate Business Park, Unit 5-7 Tintagel Way, Aldridge, Walsall WS9 8ER.
POM
If you notice any of the following serious side effects, stop using this
medicine and talk to your doctor straight away:
• severe allergic reactions such as swelling of lips, tongue and throat (may affect up to 1 in 1,000 patients treated)
• allergic reactions: skin rashes, redness, itching or weals like nettle rash and hives (may affect up to 1 in 100 patients treated)
• cough, or wheezing, which gets worse soon after taking an inhalation (may affect up to 1 in 100 patients treated)
Repackaged by MPT Pharma Ltd.
PL: 33532/0653
Leaflet dated 7th March 2016 Leaflet coded xxxxxxxxx
Alvesco® is a registered trademark of Takeda GmbH. AeroChamber Plus™ is a registered trademark of Trudell Medical International.
Ciclesonide 160 micrograms/dose inhaler CFC free
This medicine is not suitable for use in an acute attack of breathlessness. For quick relief from such an attack, use only your Reliever inhaler.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
• The name of this medicine is Ciclesonide 160 micrograms/dose inhaler CFC free but will be referred to as Ciclesonide throughout the remainder of this leaflet.
• This medicine is also available in other strengths.
What is in this leaflet:
1. What Ciclesonide is and what it is used for
2. What you need to know before you use Ciclesonide
3. How to use Ciclesonide
4. Possible side effects
5. How to store Ciclesonide
6. Contents of the pack and other information
1. What Ciclesonide is and what it is used for What Ciclesonide is:
Ciclesonide is a clear and colourless aerosol spray for you to breathe in through your mouth and into your lungs. It is a Preventer medication (corticosteroid) that has to be taken every day and which becomes active only after it has been inhaled into your lungs.
The active ingredient in this medicine is ciclesonide. (For the other ingredients, see section 6).
What Ciclesonide is used for:
This medicine is prescribed to control persistent asthma in adult and adolescent patients (12 years old and more).
It helps you to breathe more easily by decreasing the symptoms of your asthma and by lessening the chances of an asthma attack. The effect builds up over a period of time, so this medicine needs to be taken every day, even when you are feeling well.
2. What you need to know before you use Ciclesonide Do not use Ciclesonide
if you are allergic (hypersensitive) to ciclesonide or any of the other ingredients of this medicine (listed in section 6).
Take special care with Ciclesonide
• Before beginning treatment with this medicine, please tell your doctor
if:
you have ever been treated, or are currently being treated, for pulmonary tuberculosis (TB), fungal, viral or bacterial infections.
Check with your doctor if you are not sure. It is important to make sure that Ciclesonide is the right medicine for you.
During your treatment with Ciclesonide contact your doctor immediately
if.
• breathing becomes difficult and your symptoms, coughing, breathlessness, wheezing, tightness in the chest, increasing noises (rhonchi) or other symptoms of narrowing of the airways are getting worse.
(You should use your Reliever inhaler which will normally lead quickly to an improvement.)
• you are waking up at night with your symptoms.
• you are not getting relief from using your Reliever inhaler.
Your doctor will decide on your further treatment.
Specific patient groups
Patients with severe asthma are at risk of acute asthma attacks. For such patients the doctor will carry out regular thorough asthma control checks, including a lung function test.
Patients who are already taking corticosteroid tablets:
Ciclesonide can be used to replace your tablets, or to reduce the number of tablets you need to take. Please follow your doctor's instructions carefully.
• This will start about a week after you begin your Ciclesonide inhalations.
• The number of tablets you take will be reduced with caution over a period of time.
• During this period you may sometimes suffer from a general feeling of being unwell.
• In spite of this, it is important to continue both with your Ciclesonide inhalations and with slowly reducing the number of tablets you take.
• If you get serious symptoms such as nausea (feeling sick), vomiting (being sick), diarrhoea or a high temperature, contact your doctor.
• This process may sometimes reveal minor allergies such as rhinitis (inflammation of the inside of the nose) or eczema (itchy, reddening skin).
• If you have changed over from tablets, you will continue for a time to be at risk of reduced adrenal function, which is related to the corticosteroid tablets you take. The symptoms of reduced adrenal function (e.g. dizziness, fainting, nausea, loss of appetite, moodiness, decrease in body hair, inability to cope with stress, weakness, headaches, memory problems, allergies, food cravings, and blood sugar disorders) may also continue for some time.
• You may also need to see a specialist to determine the extent of the reduction in adrenal function.
• Your doctor will also do regular checks on your adrenal function.
• During periods of stress, for example, having an operation, worsening asthma attacks, it is possible that you will need extra corticosteroid tablets. If so, you must carry a steroid warning card which says so.
Patients with liver or kidney disorders:
There is no need to adjust the dose of ciclesonide if you have liver or kidney problems. If you suffer from a severe liver condition, your doctor will check you more carefully for possible side effects resulting from disturbance of normal steroid production.
Children below 12 years of age:
This medicine is not recommended for children below the age of 12 because of a lack of information about its possible effects.
Other medicines and Ciclesonide
Please inform your doctor before using Ciclesonide, if you are currently being treated for any fungal or viral infections with medicine containing:
• ketoconazole,
• itraconazole,
• ritonavir,
• nelfinavir.
These may intensify the action of Ciclesonide so that the probability of side effects cannot be completely ruled out.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using this medicine with food and drink
There is no interaction between Ciclesonide and food and drink.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant, want to become pregnant or are breast-feeding.
• Because there is not enough information about the effects of Ciclesonide on pregnant women, your doctor will discuss with you the risks and benefits of using Ciclesonide.
• Ciclesonide (the active ingredient in this medicine) may be taken during pregnancy only when the possible benefits to the mother justify the possible risk to the developing baby. If your doctor decides that you can continue using Ciclesonide, the smallest possible dose of ciclesonide will be used to maintain asthma control.
• The adrenal function will be carefully monitored in children of mothers who received corticosteroids during pregnancy.
• Talk to your doctor if you want to use Ciclesonide during breast-feeding.
• It is not known whether inhaled ciclesonide passes into the breast milk in humans.
• Prescribing Ciclesonide to women who are breast-feeding will therefore only be considered if the expected benefit to the mother outweighs the possible risk to the child.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Ciclesonide and its ingredients have no or negligible effects on the ability to drive or to use machinery.
3. How to use Ciclesonide
Always use this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
• If you have just started to use this medicine instead of, or as well as, taking corticosteroid tablets, see section 2, Patients who are already taking corticosteroid tablets.
How much Ciclesonide should I take each day?
Your doctor will have spoken to you about how much of your medicine you need to take each day. This will depend on your individual need.
• The recommended dose of Ciclesonide is 160 micrograms once daily, which leads to asthma control in the majority of patients.
• In some patients a dose reduction to 80 micrograms, once daily, may be an adequate dose for maintaining effective control of their asthma.
• An increased dosage of Ciclesonide may become necessary for a short period of time in patients who suffer a severe worsening of their asthma symptoms. This can be up to 640 micrograms per day, delivered as 320 micrograms twice daily but no data confirming the additional therapeutic effect after 3 months with these higher doses are available.
If necessary, your doctor may also prescribe corticosteroid tablets and/or, in the case of an infection, an antibiotic.
• Your doctor will adjust your dose to the minimum necessary to control your asthma.
• You should start to notice an improvement in your symptoms (wheezing, tight chest and coughing) within 24 hours.
When should I use my Ciclesonide inhaler?
In most cases, either in the morning or in the evening - as one or two puffs once a day. Follow your doctor's instructions very carefully. It is important that you take Ciclesonide regularly every day, even if you feel better.
If you find that you have to use your Reliever inhaler more than 2-3 times a week, you should contact your doctor to have your medicine reviewed.
How do I use my Ciclesonide inhaler?
It is important that a doctor, nurse or pharmacist shows you first how to use your Ciclesonide inhaler properly. A good technique will make sure you are receiving the correct amount into your lungs. Please use the instructions in this leaflet as a reminder.
You may wish to practise in front of the mirror for the first couple of times until you are confident that you are using your Ciclesonide inhaler properly. Make sure that none of your medicine is escaping from the top or sides of your mouth.
If you have a new inhaler, or if you have not used your inhaler for a week or more, it must be tested before you use it. Remove the mouthpiece cover and press down three times on the canister inside the inhaler to release three puffs into the air - away from you.
You do not need to shake your Ciclesonide inhaler before using it. The medicine is already in a very fine solution, mixed to ensure you receive the correct dose with each puff.
1. Remove the mouthpiece cover and check the mouthpiece, both the inside and the outside, to make sure that it is both clean and dry.




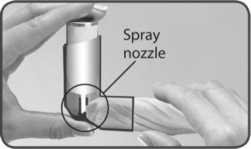
Follow these instructions carefully and use the pictures to guide you.
2. Hold the inhaler upside down (base of the canister at the top) with your forefinger on the base of the canister and your thumb under the mouthpiece.
3. Breathe out as far as is comfortable. Do not breathe out through the inhaler.
4. Place the mouthpiece in your mouth and close your lips firmly around it.
5. Just after starting to breathe in through your mouth, press down with your forefinger on the top of the inhaler to release a puff of the medicine while you are still breathing in slowly and deeply. Please take care that the puff of medicine does not escape through the top, bottom or sides of your mouth.
6. Hold your breath, take the inhaler from your mouth and remove your finger from the top of the inhaler. Continue holding your breath for about ten seconds or as long as is comfortable. Breathe out slowly through your mouth. Do not breathe out through the inhaler.
It is important that you do not rush
steps 3 to 6.
7. If you have been instructed to take another puff, wait about half a minute and repeat steps 3 to 6.
8. After use, always replace the mouthpiece cover to keep out dust. Replace firmly and snap into position.
9. For hygiene reasons
• please clean the mouthpiece weekly with a dry tissue, both inside and out.
• using a dry, folded tissue, wipe over the front of the small hole where the medicine comes out.
• do not use water or any other liquids.
A correct technique will ensure the right amount of Ciclesonide is getting into your lungs every time you use your inhaler. Your doctor will check your inhalation technique regularly to ensure that your treatment can have the very best effect.
When the canister is completely empty you will not feel or hear any of the propellant being discharged.
If you begin to feel wheezy or tightness in the chest after using your Ciclesonide inhaler:
• do not take any more puffs.
• use your reliever inhaler to help your breathing.
• contact your doctor immediately.
If you find it difficult to use the inhaler, your doctor may recommend the use of a spacer. The spacer that fits the Ciclesonide inhaler is called AeroChamber Plus™. If you use the AeroChamber Plus™ device, please follow the instructions provided with it. Your doctor or pharmacist will be able to advise you about the device.
If you use more Ciclesonide than you should
It is important that you take your dose as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
There is no specific treatment necessary if you have used too much Ciclesonide but you should inform your doctor. If high doses are used over long periods, a certain degree of reduction in adrenal function cannot be ruled out and control of the adrenal function may be necessary.
If you forget to use Ciclesonide
If you have forgotten to use your Ciclesonide, just take your usual dose when it is next due. Do not take a double number of puffs to make up for the forgotten dose.
The other side effects seen with Ciclesonide are usually mild. In most cases you can continue with your treatment. The side effects you may experience are:
Uncommon side effects (may affect up to 1 in 100 patients treated):
• hoarseness
• burning, inflammation, irritation of mouth or throat
• oral thrush (oral fungal infection)
• headache
• bad taste
• dryness of mouth or throat
• nausea or vomiting
Rare side effects (may affect up to 1 in 1,000 patients treated):
• sensation of heartbeat (palpitations)
• discomfort or pain in the abdomen
• high blood pressure
Frequency not known, but may also occur:
• Sleeping problems, depression or feeling worried, restless, nervous, over-excited or irritable. These effects are more likely to occur in children
Ciclesonide may affect the normal production of corticosteroids in your body. This is usually seen in patients taking high doses over a long period of time. These effects may include:
• reduced rate of growth in adolescents
• a thinning of the bones
• possible clouding of the lens of the eye (cataracts) causing blurred vision
• loss of vision caused by abnormally high pressure in the eye (glaucoma)
• moon-shaped face, weight gain in the upper body and thinning arms and legs (Cushingoid features or Cushing syndrome).
Adolescents who are receiving treatment for a long period of time should have their height checked regularly by their doctor. If your growth rate is slowed, your doctor will adjust your treatment if possible to the lowest dose at which effective control of asthma is maintained.
Corticosteroid tablets can lead to more side effects than a corticosteroid inhaler such as Ciclesonide. If you have been taking steroid tablets before or during the use of Ciclesonide, the risk of side effects from the tablets may continue for a period of time. Regular check-ups with your doctor will ensure that you are taking the right dose of Ciclesonide for you. Regular check-ups will also identify any side effects early on and reduce the chances of them worsening.
Please remember:
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Ciclesonide
• Keep out of the sight and reach of children.
• Do not use your inhaler after the expiry date which is stated on the label and the carton after EXP. The expiry date refers to the last day of that month.
• The container contains a pressurised liquid. Do not expose to temperatures higher than 50°C.
• The container should not be punctured, broken or burned even if it seems empty.
• As with most inhaled medicines in pressurised containers, the healing effect of this medicinal product may become smaller when the container is cold. However, Ciclesonide delivers the same level of dose from minus 10°C to plus 40°C.
• If your medicine shows any sign of deterioration, return it to your pharmacist.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information What Ciclesonide contains
Each actuation releases a puff (dose delivered through the mouthpiece) that contains 160 micrograms of the active ingredient ciclesonide.
The other ingredients are anhydrous ethanol and propellant HFA-134a (norflurane).
What Ciclesonide looks like and contents of the pack
Ciclesonide is a clear and colourless liquid in a pressurised aluminium container fitted with a plastic metering valve, mouthpiece and a brown-reddish body and cap which delivers an accurately measured dose of ciclesonide in the form of a spray.
If you stop using Ciclesonide
Even if you feel better, you should not stop using your Ciclesonide inhaler.
Ciclesonide comes in packs of 1 or 2 inhalers containing 60 metered actuations in each inhaler.
POM
If you do stop using this medicine, you must tell your doctor immediately.
4. Possible side effects
Like all medicines, Ciclesonide can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop using this medicine and talk to your doctor straight away:
• severe allergic reactions such as swelling of lips, tongue and throat (may affect up to 1 in 1,000 patients treated)
• allergic reactions: skin rashes, redness, itching or weals like nettle rash and hives (may affect up to 1 in 100 patients treated)
• cough, or wheezing, which gets worse soon after taking an inhalation (may affect up to 1 in 100 patients treated)
Manufactured by
Takeda GmbH, Byk-Gulden-Str. 2, D-78467 Konstanz, Germany
Procured from within the EU by the Product Licence Holder:
MPT Pharma Ltd, Westgate Business Park, Unit 5-7 Tintagel Way, Aldridge, Walsall WS9 8ER.
Repackaged by MPT Pharma Ltd.
PL: 33532/0653
Leaflet dated 7th March 2016 Leaflet coded xxxxxxxxx
AeroChamber Plus™ is a registered trademark of Trudell Medical International.