Combivent Udvs
Out of date information, search anotherPackage leaflet: Information for the user
Combivent® UDVs®
500 microgram/2.5mg per 2.5ml Nebuliser Solution
(ipratropium bromide and salbutamol sulphate)
Read all of this leaflet carefully before you
start taking this medicine
• Keepthis leaflet. You mavneedto read it again.
• If you have anyfurtherquestions, askyour doctor, pharmacist or practice nurse.
• This medicine has been prescribedfor you. Do not pass it on to others. It may harm them, even if theirsymptoms are the same as yours.
• If any of the side effects gets troublesome or serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In {\)\$ IgsTIgI*
1. What COMBIVENT UDVs is and what it is
usedfor
2. Before you use COMBIVENT UDVs
3. Howto use COMBIVENT UDVs
4. Possible side effects
5. Howto store COMBIVENT UDVs
6. Further information
1. WHAT COMBIVENT UDVs IS AND WHAT IT IS USEDFOR
The name of your medicine is COMBIVENT UDVs. You use it with a device called a :nebuliser'. This changes your medicine into a mistforyou to breathe in. COMBIVENT contains two different medicines called:
• Ipratropium bromide and
• Salbutamol sulphate
Both belong to a group of medicines called bronchodilators. They are used to make breathing easierin an illness called ‘chronic obstructive pulmonary disease’ orCOPD. They work by opening up your airways.
2. BEFORE YOU USE COMBIVENTUDVs
Do not use COMBIVENT if:
• You are allergic(hypersensitive)to
ipratropium orsalbutamol orany of the other ingredients in COMBIVENT. (Listed in section 6: Further information.)
• You are allergictosimilarmedicineswhich contain atropine ormedicines like atropine
• You have a heart problem called ‘hypertrophic obstructive cardiomyopathy’. This is where the wall between the two sides of the heart gets biggerand blocks the bloodflow
• You have a very fast heart beat (called tachyarrythmia’)
• You are pregnant, likelyto get pregnantor are breast-feeding
Do not use if any of the above applyto you. If you are not sure, talk to yourdoctoror pharmacist before using COMBIVENT.
Take special care with COMBIVENT
Check with yourdoctoror pharmacist before using this medicine if:
• You have glaucoma, orhave beentold that you may develop it
• You have heart or circulation problems, or have had a recent heart attack
• You have diabetes
• You have an over-active thyroid gland
• You have problems passing water(urine)
• You are a man who has prostate problems
• You have cystic fibrosis
• You have everhad something called ‘pheochromocytoma’. This is a rare tumour which is not malignant. Using your inhaler can make the symptoms of this worse
If you are not sure if any of these applyto you. talk to your doctor or pharmacist before using COMBIVENT.
Taking other medicines Pleasetellyour doctoror pharmacist if you are
taking or have recentlytaken any other medicines, including medicines obtained
without a prescription. This includes herbal medicines. This is because COMBIVENT can affect the way some other medicines work. Also some othermedicines can affect the way COMBIVENT works.
In particular, tell yourdoctoror pharmacist if you are taking any of the following medicines:
• Steroid medicines such as prednisolone
• Water tablets (also called ‘diuretics')
• Medicinesfordepression
• Medicines to help your breathing
• Medicines called :anti-cholinergics'. These can be usedto treat colic pain, Parkinson's Disease, problems passingwaterorlack of control of your bladder or bowels
• Medicines called 'beta blockers'such as propanolol. These can be used to treat heartproblems, high blood pressure, anxietyor migraine
• Medicines called 'beta mimetics' such as fenoterolforbreathing problems
• Digoxin-usedforafastheartbeator heartfailure
If you are not sure if any of the above applyto you. talk to your doctor or pharmacist before using COMBIVENT.
Operations
Some gases used in operations (anaesthetic gases) may affect howyour medicine works.
If you are about to have surgery, make sure you mention that you are taking COMBIVENT to the doctor, dentist or anaesthetist.
Tests
If you have to provide an urine sample as part of a routine sport drug test, tell the person giving the test that you are taking this medicine. This is because COMBIVENT contains salbutamol andthis mayleadto a positive result.
Pregnancy and breast-feeding
Do not use COMBIVENT if you are pregnant, likelyto get pregnantorare breast-feeding.
Driving and using machines
You mayfeel dizzy, or have difficulty in focusing, or blurred vision whilsttaking COMBIVENT. If this happens do not drive or use tools or machines.
3. HOW TO USE COMBIVENT UDVs
Always use COMBIVENT exactly as your doctorhas told you. You should check with yourdoctor or pharmacist if you are notsure. Followthese instructions to get the best results. If anything is unclearafterreading this leaflet, askyour doctor, pharmacistor practice nurse.
How many dose units
The usual dose is the contents of 1 single
dose unit, three orfourtimes a dayfor:
Adults (including the elderly) and children over 12 years.
COMBIVENT is not recommended for children under 12 years.
Do not swallow or give this medicine by injection.
Do not use more than your doctor has told you
See your doctor straight away if:
• You feel that your medicine is not working as well as usual
• You needto use the nebulisermorethan yourdoctor has recommended
Your doctor mayneedto check how well your medicine is working.
In some cases your doctormay needto changeyourmedicine.
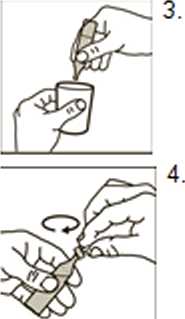
• Twist off the top
• Always hold it upright while you do'this
Howto use your nebuliser
Read through numbers 1 to 6 first, before starting to use your nebuliser.
1. Get yournebuliserreadybyfollowingthe manufacturer's instructions. Askyour doctor if you are not sure howto use it.
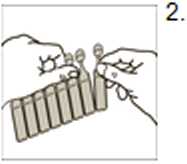
Open the pouch and remove the strip of unit dose vials Carefullyseparatea new dose unitfrom the strip
Do not use if it is already openorthe liquid inside has discoloured.
• Squeeze all the contents of the dose unit into the nebuliserchamber
• Your doctor will tell you if you need to
use a different amount
• If your doctor has told you that your medicine needs to be diluted, you will
be given'sterile sodium chloride 0.9%’solution. Your doctor will tell you howto do this
5. Use yournebuliseras directed byyour doctor.
6. • Afteryou have finished, dispose of any
leftover medicine carefully
• Followthe manufacturer's instructions on howto cleanyournebuliser
• It isimportanttokeepyournebuliser clean
If any of the liquid or mist accidentally gets into youreyesyou maygetpainful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talkto your doctorfor advice. If you get problems with your eyes at any othertime, talk to your doctorfor advice.
If you use more COMBIVENT than you should
If you use more of this medicine than you should,talkto a doctororgoto a hospital straightaway.
If you forget to take COMBIVENT
• If you forget a dose, take it as soon as you remember it
• However, if it is time forthe next dose, skip the missed dose
• Donottake a double doseto make upfor a forgotten dose
If you have anyfurtherquestions, askyour doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, COMBIVENT can cause side effects, although not everybody gets them.
Tell your doctor straight away if you notice any of the following serious side effects -you may need urgent medical treatment:
• If aftertaking COMBIVENT you are wheezy or have other difficulties in breathing, do not take any more (unless you have been told to by your doctor)
• Allergic reactions-the signs may include skin rash, itching and nettle rash. In severe cases the signs include swelling of your tongue, lips and face, sudden difficulties in breathing and a fall in your blood pressure that may cause dizziness.
See your doctor straight away if you have any of these side effects.
The side effects described below have been experienced by people taking COMBIVENT.
Uncommon (affects less than 1 in 100 people)
• Feeling nervous, shaky or dizzy
• Dry mouth
• Cough, headache
• Feeling sick(nausea)
• Throat irritation
• Increase in blood pressure
• Increased heart rate or uneven heartbeats (palpitations)
• Voice problems (‘dysphonia’)
• Skin reactions
Rare (affects less than 1 in 1,000 people)
• Irregularheartbeat
• Regular but abnormallyfast heart rate (supraventriculartachycardia)
• Chestpain(dueto heart problems such as angina). Tell your doctor or pharmacist if this occurs but do notstop taking this medicine unlesstoldto do so
• Blurred vision, dilated pupils, glaucoma, painful, stinging, or red eyes, swelling of the eyes, see colours or lights
• Increased sweating
• Unexpected tightening of the chest immediately after inhaling the medicine
• Drythroat, swelling of the throat
• Difficulty in breathing orspeaking dueto a brief spasm of yourvocal muscles
• Diarrhoea, constipation, being sick (vomiting) or other problems with your digestive system
• Inflammation of the mouth
• Muscle cramps, muscle weakness and pain
• Difficultyinpassingwater(urine)
• Feelingweak
• Fall in blood pressure
• Mood changes
You mayalsoget unusuallylowlevelsof potassium in your blood (called ’hypokalemia'). If this happens, your doctor will keep checking your potassium levels.
If any of the side effects gets troublesome or serious, or if you notice anyside effects not listed in this leaflet, tell your doctor or pharmacist.
If any of the liquid or mist accidentally gets into youreyesyou maygetpainful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talkto your doctorfor advice. If you get problems with your eyes at any othertime, talk to your doctorfor advice.
5. HOW TO STORE COMBIVENTUDVs
Do not use this medicine afterthe expiry date which is stated on the carton and the vial label. The expiry date refers to the last day of the month.
Do not store above 25°C. Do not freeze. Do not use if the solution is discoloured. Protect from light.
Keepvials in the outercarton in orderto protect from light.
Keep out ofthe sight and reach of children.
6. FURTHER INFORMATION
What COMBIVENT contains
COMBIVENT UDVs contain a nebuliser solution. Each single dose unit contains ipratropium bromide 500 micrograms and salbutamol 2.5 mg (as sulphate) in 2.5 ml of isotonic preservative free solution.
The other ingredients are: sodium chloride, hydrochloric acid and purified water.
What COMBIVENT looks like and contents of the pack
Your medicine comes with a nebuliser. This changes COMBIVENT into a mistforyouto breathe in. COMBIVENT UDVs are available in packs of 60 units.
Manufacturer
COMBIVENT UDVs are manufactured by: Laboratoire Unither, Zl de Longpre, 10 rue Andre Durouchez, 80084 Amiens Cedex2, France
Procured from within the EU byproduct Licence holder: PilsCo Ltd., 9-15 Springburn Place, East Kilbride, G74 5NU.
This medicine has been repackaged by TBC
This leafletwas revised 30/08/2012. Combivent and UDVs are registered trademarks of Boehringer Ingetheim Pharma GmbH & Co. KG