Donepezil Hydrochloride 5 Mg Orodispersible Tablets
Out of date information, search anotherIteva uk Ref: 231-30-17704-D LEA DONEPEZIL A/S OD TAB TUK <DEB
Version: 4 29 May 2015
Pharma Code 1885 Pharma Code 1885
DONEPEZIL HYDROCHLORIDE 5 mg ORODISPERSIBLE TABLETS DONEPEZIL HYDROCHLORIDE 10 mg ORODISPERSIBLE TABLETS
PACKAGE LEAFLET: INFORMATION FOR THE USER
You and your caregiver should read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet. See section 4.
1. What Donepezil Hydrochloride is and what it is used for
2. Before you take Donepezil Hydrochloride
3. How to take Donepezil Hydrochloride
4. Possible side effects
5. How to store Donepezil Hydrochloride
6. Further information
OWHAT DONEPEZIL
HYDROCHLORIDE IS AND WHAT IT IS USED FOR
Donepezil hydrochloride belongs to a group of medicines called acetylcholine esterase inhibitors: It increases the levels of the substance acetylcholine in the brain involved in memory function by slowing down the breakdown of acetylcholine.
It is used to relieve the symptoms of mild to moderately severe Alzheimer's dementia. The symptoms include increasing memory loss, confusion and behavioural changes. As a result, sufferers of Alzheimer's disease find it more and more difficult to carry out their normal daily activities.
©BEFORE YOU TAKE DONEPEZIL HYDROCHLORIDE
Remember: You must tell your doctor who your carers are.
DO NOT TAKE Donepezil Hydrochloride
• if you are allergic (hypersensitive) to donepezil hydrochloride or any of the other ingredients of Donepezil Hydrochloride listed in section 6.
• if you are allergic (hypersensitive) to other medicines containing a piperidine derivative (donepezil is a piperidine derivative).
Take special care with Donepezil Hydrochloride
Treatment with Donepezil Hydrochloride should only be started and supervised by a doctor with experience in diagnosing and treating Alzheimer's dementia.
Inform your doctor if you:
• have ever had a stomach or intestinal ulcer
• frequently take pain-killers or treatment for rheumatism (pain or inflammation around bones, joints or muscles): taking these medicines at the same time as Donepezil Hydrochloride could put you at greater risk of developing stomach or intestinal ulcers.
• have ever had seizures • have a heart condition (irregular or very slow heart beat)
• have asthma or other long-term lung disease
• have difficulties when urinating • have ever had any liver problems or hepatitis
• are going to have an operation that requires you to have a general anaesthetic. You must inform the anaesthetist that you are taking Donepezil Hydrochloride. This is because your medicine may affect the amount of anaesthetic needed.
Also tell your doctor if you are pregnant or think you might be pregnant.
Donepezil Hydrochloride can be used in patients with kidney disease or mild to moderate liver disease. Tell your doctor first if you have kidney or liver disease. Patients with severe liver disease should not take Donepezil Hydrochloride.
The individual effect of Donepezil Hydrochloride cannot be predicted, therefore the effect of the treatment should be evaluated regularly by a doctor.
Children and adolescents
Donepezil hydrochloride is not recommended in children and teenagers under 18 years of age.
Taking other medicines
Inform your doctor if you are being treated with any of the following medicines, because the effect of Donepezil Hydrochloride or the other medicine could be influenced if you take the two medicines together:
• Medicines against fungal infections, such as ketoconazole or itraconazole
• Pain killers or treatment for arthritis e.g. aspirin, non-steroidal anti-inflammatory (NSAID) drugs such as ibuprofen, or diclofenac sodium
• Anticholinergics medicines, such as tolterodine
• Antibiotics, such as erythromycin or rifampicin
• Heart medicines, such as quinidine or beta-blockers
• Medicines for epilepsy, such as phenytoin or carbamazepine
• Antidepressants, such as fluoxetine
• Muscle relaxants
• General anaesthetic
• Other medicines that act the same way as Donepezil Hydrochloride (such as galantamine or rivastigmine), and some medicines for diarrhoea, Parkinson's disease or asthma
• medicines obtained without a prescription, such as herbal remedies.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription and herbal remedies.
Tell your doctor or pharmacist the name of your caregiver. Your caregiver will help you to take your medicine as it is prescribed.
Taking Donepezil Hydrochloride with food and drink
Food will not influence the effect of Donepezil Hydrochloride.
You must avoid drinking alcohol while you are being treated with Donepezil Hydrochloride, because it could reduce the effect of Donepezil Hydrochloride.
Pregnancy and breast-feeding
You should not use Donepezil Hydrochloride if you are pregnant or breast-feeding.
If you are pregnant, or think you might be pregnant, ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Alzheimer's disease may impair your ability to drive or operate machinery. You should not drive unless your doctor tells you that it is safe to do so. Also, your medicine can cause tiredness, dizziness and muscle cramp and if affected, you must not drive or operate machinery.
Important information about some of the ingredients of Donepezil Hydrochloride
Donepezil Hydrochloride contains lactose.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
This medicinal product contains aspartame, a source of phenylalanine and may be harmful for people with phenylketonuria.
a
HOW TO TAKE DONEPEZIL HYDROCHLORIDE
Always take Donepezil Hydrochloride exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. The usual dose is described below.
The tablet strength you will take may change depending on the length of time you have been taking the medicine and on what your doctor will recommend. For doses not realisable/practicable with this strength other strengths of this medicinal product are available.
Dosage
Usually, you will start by taking 5 mg donepezil hydrochloride (one white tablet) every night. After one month, your doctor may tell you to take 10 mg donepezil hydrochloride (one yellow tablet) every night. The maximum recommended dose is 10 mg donepezil hydrochloride each night.
Both you and your caregivers should be aware of the doctor's instructions.
Take your tablet by mouth at night before you go to bed:
• Do not push the tablet out of the pocket, as this will crush it
• Each strip contains tablets separated in pockets by perforations. Tear off one tablet pocket along the dotted lines (Figure 1)
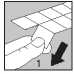
• Carefully peel off the lidding foil, starting in the corner indicated by the arrow (Figures 2 and 3)
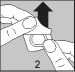
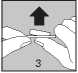
• Keep your hands dry and take the tablet out of the strip.
• The tablet should be placed on the tongue and allowed to disintegrate before swallowing with or without water, according to your preference.
For how long should you take Donepezil Hydrochloride?
Your doctor or pharmacist will advise you on how long you should continue to take your tablets.You will need to see your doctor from time to time to review your treatment and assess your symptoms.
If you take more Donepezil Hydrochloride than you should
DO NOT take more than one tablet each day. Contact your doctor or nearest hospital immediately if you take too high a dose of Donepezil Hydrochloride. At a high dose (overdose), the listed side effects may be worse (see section 4: Possible side effects). In particular:
• severe nausea (feeling sick)
• vomiting (being sick)
• drooling
• sweating
• a slow heart beat
• low blood pressure (light-headedness or dizziness when standing)
• difficulty breathing
• collapse (losing consciousness)
• seizures and muscle weakness may occur. Always take the tablets and the carton with you to the hospital so that the doctor knows what has been taken.
If you forget to take Donepezil Hydrochloride
If you forget to take a dose, take it as quickly as possible after noticing this, unless it is time for the next dose.
Do not take a double dose to make up for a forgotten dose. Take your customary dose on the following day at the usual time.
If you forget to take your medicine for more than one week, call your doctor before taking any more medicine.
If you stop taking Donepezil Hydrochloride
Do not stop taking the tablets unless told to do so by your doctor. If you stop taking Donepezil Hydrochloride, the benefits of your treatment will gradually fade away.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
^4 POSSIBLE SIDE EFFECTS
Like all medicines, Donepezil Hydrochloride can cause side effects, although not everybody gets them.
Serious side effects:
You must tell your doctor immediately if you notice these serious side effects mentioned. You may need urgent medical treatment.
• liver damage e.g. hepatitis. The symptoms of hepatitis are feeling or being sick, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin and eyes, and dark coloured urine (affects 1 to 10 users in 10,000)
• stomach or duodenal ulcers. The symptoms of ulcers are stomach pain and discomfort (indigestion) felt between the navel and the breast bone (affects 1 to 10 users in 1,000).
• bleeding in the stomach or intestines. This may cause you to pass black tar like stools or visible blood from the rectum (affects 1 to 10 users in 1,000).
• seizures (fits) or convulsions (affects 1 to 10 users in 1,000).
• fever with muscle stiffness, sweating or a lowered level of consciousness (a disorder called "Neuroleptic Malignant Syndrome") (affects up to 1 user in 10,000).
Contact your doctor if you experience the following side effects:
• hallucinations
• agitation
• aggressive behaviour
• convulsions or brief fainting episodes as the dose might need to be lowered or treatment stopped.
Very common side effects (which affect more than 1 user in 10) are diarrhoea, nausea and headache.
Common side effects (which affect 1 to 10 users in 100) are dizziness, sleeplessness, tiredness, fainting, hallucinations, unusual dreams including nightmares, agitation, aggressive behaviour, pain, loss of appetite, digestive disturbances, including vomiting, incontinence, muscle cramps, rash, itching, more prone to colds and having accidents.
Uncommon side effects (which affect 1 to 10 users in 1,000) are slow heart rate, and abnormal levels of the substance creatine kinase in the blood.
Rare side effects (which affect 1 to 10 users in 10,000) are heart problems like abnormal heart rate, as well as symptoms such as shaking, stiffness or uncontrollable movement of the face and tongue, but also of the limbs.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
©HOW TO STORE DONEPEZIL HYDROCHLORIDE
Keep out of the reach and sight of children.
Do not use Donepezil Hydrochloride after the expiry date which is stated on the blister and carton after EXP. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.These measures will help to protect the environment.
FURTHER INFORMATION
What Donepezil Hydrochloride Orodispersible Tablets contain
The active substance is donepezil hydrochloride.
5 mg: Each orodispersible tablet contains 5 mg donepezil hydrochloride equivalent to 4.56 mg donepezil.
10 mg: Each orodispersible tablet contains 10 mg donepezil hydrochloride equivalent to 9.12 mg donepezil.
The other ingredients are mannitol (E421), hypromellose, silical colloidal anhydrous, crospovidone, lactose monohydrate, maize starch, aspartame (E951), magnesium stearate.
10 mg orodispersible tablet also contains:
iron oxide, yellow.
What Donepezil Hydrochloride Orodispersible Tablets look like and contents of the pack
Orodispersible tablet 5 mg: White, flat bevelled edge, round tablet, engraved with "L 5” on one side and plain on the other, available in pack sizes of 1, 7, 28, 30, 50, 56, 60, 98, 100 or 120 orodispersible tablets.
10 mg: Yellow, flat bevelled edge, round tablet engraved with "L 10” on one side and plain on the other, available in pack sizes of 1, 7 28, 30, 50, 56, 60, 98, 100 or 120 orodispersible tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Teva UK Limited, Eastbourne, BN22 9AG
Manufacturer
TEVA Pharmaceutical Works Private Ltd, Company, Pallagi ut 13, 4042 Debrecen, Hungary
This leaflet was last revised in May 2015
PL 00289/1135 PL 00289/1136
17704-D
20063670