Dorzolamide 20 Mg/Ml Eye Drops Solution
Out of date information, search anotherRead all of this leaflet carefully before you start using this medicine because it contains important information for you.
• keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
DORZOLAMiDE 20 mg/ml EYE DROPS, SOLUTiON
(dorzolamide)
What is in this leaflet:
1. What Dorzolamide is and what it is used for
2. What you need to know before you use Dorzolamide
3. How to use Dorzolamide
4. Possible side effects
5. How to store Dorzolamide
6. contents of the pack and other information
1. WHAT DoRzoLAMiDE is AND WHAT iT is
used for
Dorzolamide is supplied as a sterile eye drop solution. Dorzolamide contains dorzolamide, a sulfonamide-related compound, as the active ingredient.
Dorzolamide belongs to a group of medicines called ophthalmic carbonic anhydrase inhibitors which reduce high pressure in the eye.
Dorzolamide is used to lower raised pressure in the eye and to treat glaucoma (open-angle glaucoma, pseudo-exfoliative glaucoma). Dorzolamide can be used alone or in addition to other medicines which lower the pressure in the eye (so-called beta-blockers).
2. what you need to know before you use dorzolamide
Do not use Dorzolamide:
• if you are allergic to dorzolamide or any of the other ingredients of this medicine (listed in section 6)
• if you have severe kidney problems.
• if you have a disturbance in the pH (acid/alkali balance) of your blood.
Warnings and precautions - Talk to your doctor or pharmacist before using Dorzolamide if:
• if you have, or have had, liver problems in the past
• if you have been told you have a corneal defect
• if you have had any allergies to any sulfonamide like medicines e.g. co-trimoxazole
• if you have had, or are about to have, eye surgery
• if you have suffered an eye injury or very low pressure in the eye (hypotony)
• if you have a prior history of kidney stones
• if you are taking another carbonic anhydrase inhibitor by mouth e.g acetazolamide
• if you wear contact lenses (see the section 'Dorzolamide contains the preservative benzalkonium chloride').
During treatment
You should contact your doctor immediately if you develop any eye irritation or any new eye problems such as redness of the eye or swelling of the surface layer of the eye or eyelids.
Stop using Dorzolamide and contact your doctor immediately if you suspect that Dorzolamide is causing an allergic reaction (for example, skin rash or itching, or inflammation of the eye).
Children and adolescents - Dorzolamide should only be used in children and adolescents if the benefits outweigh the risks. Your doctor will be able to advise you.
other medicines and Dorzolamide - Tell your doctor or pharmacist if you are taking or using, or have recently taken or used, or might take or use any other medicines., including medicines obtained without a prescription.
In particular you should tell your doctor if you are taking another carbonic anhydrase inhibitor by mouth such as acetazolamide.
Pregnancy, breast-feeding - If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Dorzolamide should not be used during pregnancy unless your doctor still recommends it.
Dorzolamide should not be used while breast-feeding.
Driving and using machines - Dorzolamide may cause dizziness and visual disturbances in some patients. Do not drive or use any tools or machines until the symptoms have cleared.
Dorzolamide contains the preservative benzalkonium chloride
• Benzalkonium chloride may cause eye irritation.
• Benzalkonium chloride is known to discolour soft contact lenses.
• Avoid contact with soft contact lenses.
• remove contact lenses prior to application and wait until 15 minutes before reinsertion.
3. how to use dorzolamide
Always use this medicine exactly as your doctor or pharmacist has told you. check with your doctor or pharmacist if you are not sure.
The appropriate dosage and duration of treatment will be established by your doctor.
When Dorzolamide is used alone, the recommended dose is one drop in the affected eye(s) three times a day, for example in the morning, in the afternoon and in the evening.
If your doctor has recommended you use Dorzolamide with a beta-blocker eye drop (other medicines which lower the pressure of the eye), then the recommended dose is one drop of Dorzolamide in the affected eye(s) two times a day, for example in the morning and in the evening.
If you use Dorzolamide with another eye drop, leave at least 10 minutes between putting in Dorzolamide and the other medicine.
If you are about to start using Dorzolamide to replace another eye drop medicine, used to lower eye pressure, you should stop using the other medicine after taking the proper dosing on one day, and start Dorzolamide on the next day.
Do not change the dosage of this medicine without talking to your doctor. If you must stop treatment, contact your doctor immediately.
Do not allow the tip of the container to touch your eye or areas around your eye. It may become contaminated with bacteria that can cause eye infections that may lead to serious damage of the eye, or even loss of vision. To avoid possible contamination of the container, keep the tip of the container away from contact with any surface.
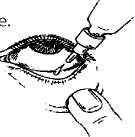
instructions for use:
It is recommended that you wash your hands before putting in your eye drops.
It may be easier to apply your eye drops in front of a mirror.
1. Before using the medication for the first time, be sure that the tamper-proof seal on the bottle neck is unbroken. A gap between the bottle and the cap is normal for an unopened bottle.Then breakthe seal.
2. Take off the cap of the bott
3. Tilt your head back and gently pull your lower eyelid down to form a small pocket between your eyelid and your eye.
4. Invert the bottle, and squeeze it until a single drop is dispensed into the eye as directed by your doctor. Do NoT TOUGH YoUR EYE oR EYELID WITH THE DRoPPER TIP.
5. Repeat steps 3 & 4 with the other eye if instructed to do so by your doctor.
6. Put the cap back on and close the bottle straight after you have used it.
if you use more Dorzolamide than you should
If you put too many drops in your eye or swallow any of the contents, you should contact your doctor immediately.
if you forget to use Dorzolamide
It is important to use Dorzolamide as prescribed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to your regular dosing schedule.
Do not use a double dose to make up for a forgotten dose.
if you stop using Dorzolamide
Dorzolamide should be used every day to work properly. If you must stop treatment, contact your doctor immediately.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSiBLE SiDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
stop using Dorzolamide immediately and go straight away to hospital or seek medical advice from your doctor if you get any of the following side effects:
Common (may affect up to 1 in 10 people):
• Damage to the front layer of the eye, which may cause eye pain, redness of the eye and blurred vision, with possible sensitivity to light, or a feeling that something is in the eye (superficial punctate keratitis).
uncommon (may affect up to 1 in 100 people):
• Swelling of the iris (the coloured part of the eye) which may cause blurred vision and seeing dark spots that appear to float across the eye, or possible flashing lights (iridocyclitis).
Rare (may affect up to 1 in 1000 people):
• Allergic reactions that may appear as a very itchy or sudden rash, sudden swelling of the eye, eyelid, lips, tongue, face, hands or throat which may cause difficulty breathing and wheezing.
• Excessive painful redness of the skin, large blisters, skin peeling off in sheets, bleeding of the lips, eyes, genitals or mouth accompanied by fever (this may indicate Stevens-Johnson syndrome or toxic epidermal necrolysis)
• Sudden loss of vision, accompanied by pain and sometimes feeling and being sick which may occur after eye surgery
(choroidal detachment)
• A large decrease in the pressure of the eye, which can lead to eye problems such as loss of vision or the vision may become distorted
other side effects include:
Very common (may affect more than 1 in 10 people):
• Burning and stinging sensations of the eyes
Common (may affect up to 1 in 10 people):
• Headache
• Increased tear production
• Inflammation or infection of the eye
• Inflammation or crusting of the eyelid
• Itching of the eye
• Eyelid irritation
• Blurred vision (without other side effects)
• Feeling sick
• Bitter taste sensation
• Weakness or feeling tired
Rare (may affect up to 1 in 1000 people):
• Dizziness
• Tingling of hands and/or feet
• Irritation of the eyes which may cause redness and eye pain
• Difficulty in focussing on distant objects (myopia) which normally resolves when treatment is stopped
• Swelling of the front layer of the eye which you may notice as blurred vision, possibly with appearance of halos around objects (corneal oedema).
• Nosebleeds
• Irritated throat
• Dry mouth
• Itchy and scaly skin, sometimes with burning and stinging, which may also occur in the skin surrounding the eye and maybe a sign of an allergic reaction in the skin
• Kidney stones
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. how to store dorzolamide
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle label and the carton after 'EXP': The expiry date refers to the last day of that month.
Keep the bottle in the outer carton in order to protect from light. Store below 30°C. Dorzolamide should be used within 28 days after the bottle is first opened. Therefore, you must throw away the bottle 4 weeks after you first opened it, even if some solution is left. To help you remember, write down the date that you opened it in the space on the carton.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. contents of the pack and other information
What Dorzolamide contains
• The active substance is dorzolamide. Each ml contains 20 mg dorzolamide (as dorzolamide hydrochloride).
• The other ingredients are benzalkonium chloride (as a preservative) (see section 2), mannitol, hydroxyethylcellulose, sodium citrate, sodium hydroxide for pH adjustment and water for injection.
What Dorzolamide looks like and contents of the pack
Dorzolamide is a sterile, pH, buffered, colourless, slightly viscous solution in a white plastic bottle with a sealed dropper tip and a two-piece tamper proof cap assembly. Each bottle contains 5 mL of the eye drop solution. Dorzolamide is available in packs containing 1 bottle, 3 bottles or 6 bottles.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Mylan, Potters Bar, Hertfordshire, EN6 1TL,
United Kingdom
Manufacturers
Pharmathen S.A., Dervenakion 6, Pallini 15331, Attiki, Greece.
Famar SA - 63 Agiou Dimitriou Street, Alimos, Athens, 174-56 Greece
Mc Dermott Laboratories Limited t/a Gerard Laboratories - 35 Baldoyle Industrial Estate, Grange Road, Dublin 13, Ireland
Generics (UK) - Station Close, Potters Bar, Hertfordshire, EN6 1AG, United Kingdom
This leaflet was last revised in June 2014
446370