Dorzolamide 20mg/Ml Eye Drops Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Dorzolamide 20 mg/ml Eye Drops, Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active substance: One ml contains 20 mg dorzolamide (as 22.3 mg of dorzolamide hydrochloride)
Excipient: 0.08 mg benzalkonium chloride/ml For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Eye drops, solution
Isotonic, buffered, slightly viscous, aqueous solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Dorzolamide is indicated:
• as adjunctive therapy to beta-blockers,
• as monotherapy in patients unresponsive to beta-blockers or in whom beta-blockers are contraindicated, in the treatment of elevated intra-ocular pressure in:
• ocular hypertension,
• open-angle glaucoma,
• pseudo-exfoliative glaucoma.
4.2 Posology and method of administration
When used as monotherapy, the dose is one drop of dorzolamide in the conjunctival sac of the affected eye(s), three times daily.
When used as adjunctive therapy with an ophthalmic beta-blocker, the dose is one drop of dorzolamide in the conjunctival sac of the affected eye(s), two times daily.
When substituting dorzolamide for another ophthalmic anti-glaucoma agent, discontinue the other agent after proper dosing on one day, and start dorzolamide on the next day.
If more than one topical ophthalmic drug is being used, the drugs should be administered at least ten minutes apart.
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures.
Patients should also be instructed that ocular solutions, if handled improperly, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Patients should be informed of the correct handling of Dorzolamide eye drops solution.
Usage instructions
Please follow these instructions carefully when using Dorzolamide eye drops solution. It is recommended that you wash your hands before putting in your eye drops.
1. You must not use the bottle if the tamper-proof seal on the bottle neck is broken before you first use it.
2. To open the bottle, unscrew the cap by turning it until the tamper-proof seal breaks.
3. Tilt your head back and pull your lower eyelid down slightly to form a pocket between your eyelid and your eye (Fig. 1).
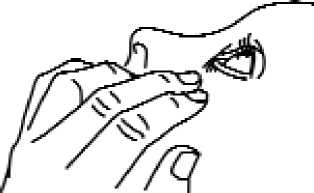
4. Invert the container, and press gently as shown (Fig. 2) until a single drop as instructed by your doctor is dispensed into your eye. DO NOT TOUCH YOUR EYE OR EYELID WITH THE TIP OF THE CONTAINER.
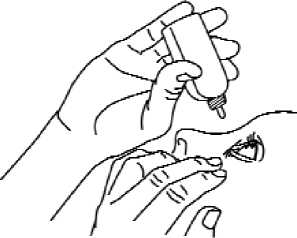
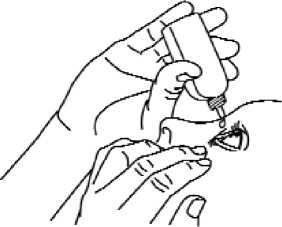
5. Repeat steps 3 and 4 with the other eye if instructed to do so by your doctor.
6. Re-close the bottle by turning the cap firmly immediately after use and return the bottle to the original outer carton.
7. The dispenser tip is designed to provide a pre-measured drop; therefore, do not enlarge the hole of the dispenser tip.
Paediatric use
The experience in children is limited. Limited clinical data in paediatric patients with administration of dorzolamide three times a day are available. (For information regarding paediatric dosing see section 5.1).
4.3 Contraindications
Dorzolamide is contraindicated in patients who are hypersensitive to the active substance or to any of the excipients.
Dorzolamide has not been studied in patients with severe renal impairment (CrCl < 30 ml/min) or with hyperchloraemic acidosis. Because dorzolamide and its
metabolites are excreted predominantly by the kidney, dorzolamide is therefore contraindicated in such patients.
4.4 Special warnings and precautions for use
Dorzolamide has not been studied in patients with hepatic impairment and should therefore be used with caution in such patients.
The management of patients with acute angle-closure glaucoma requires therapeutic interventions in addition to ocular hypotensive agents. Dorzolamide has not been studied in patients with acute angle-closure glaucoma.
Dorzolamide is a sulphonamide and although administered topically, is absorbed systemically. Therefore the same types of adverse reactions that are attributable to sulphonamides may occur with topical administration. If signs of serious reactions of hypersensitivity occur, discontinue the use of this preparation.
Therapy with oral carbonic anhydrase inhibitors has been associated with urolithiasis as a result of acid-base disturbances, especially in patients with a prior history of renal calculi. Although no acid-base disturbances have been observed with dorzolamide, urolithiasis has been reported infrequently. Because dorzolamide is a topical carbonic anhydrase inhibitor that is absorbed systemically, patients with a prior history of renal calculi may be at increased risk of urolithiasis while using dorzolamide.
If allergic reactions (e.g. conjunctivitis and eyelid reactions) are observed, discontinuation of treatment should be considered.
There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and dorzolamide. The concomitant administration of dorzolamide and oral carbonic anhydrase inhibitors is not recommended.
Corneal oedemas and irreversible corneal decompensations have been reported in patients with pre-existing chronic corneal defects and/or a history of intraocular surgery while using dorzolamide. Topical dorzolamide should be used with caution in such patients.
Choroidal detachment concomitant with ocular hypotony have been reported after filtration procedures with administration of aqueous suppressant therapies.
Dorzolamide contains the preservative benzalkonium chloride, which may cause eye irritation. Contact lenses should be removed prior to application and wait at least 15 minutes before reinsertion. Benzalkonium chloride is known to discolour soft contact lenses.
Paediatric patients:
Dorzolamide has not been studied in patients less than 36 weeks gestational age and less than one week of age. Patients with significant renal tubular immaturity should only receive dorzolamide after careful consideration of the risk benefit balance because of the possible risk of metabolic acidosis.
4.5 Interaction with other medicinal products and other forms of interaction
Specific drug interaction studies have not been performed with dorzolamide.
In clinical studies, dorzolamide was used concomitantly with the following medications without evidence of adverse interactions: timolol ophthalmic solution, betaxolol ophthalmic solution and systemic medications, including ACE-inhibitors, calcium-channel blockers, diuretics, non-steroidal anti-inflammatory drugs including acetylsalicylic acid, and hormones (e.g. oestrogen, insulin, thyroxine).
Association between dorzolamide and miotics and adrenergic agonists has not been fully evaluated during glaucoma therapy.
Dorzolamide is a carbonic anhydrase inhibitor and, although administered topically, it is also absorbed into the systemic circulation. In clinical studies, no acid-base disturbances have been observed in connection with dorzolamide use. However, such effects have been reported with oral carbonic anhydrase inhibitors, and in some cases they have resulted in medicine interactions (e.g. toxic reactions in patients receiving high doses of salicylates). The potential for such interactions should therefore be considered in association with dorzolamide therapy.
4.6 Fertility, pregnancy and lactation
There are no adequate data from the use of dorzolamide in pregnant women. Studies in rabbits have shown teratogenic effect at maternotoxic doses (see section 5.3).
Dorzolamide should not be used during pregnancy.
Lactation: There are no data showing whether the drug is excreted in human milk. Dorzolamide should not be used during lactation. In lactating rats, decreases in the body weight gain of offspring were observed.
4.7 Effects on ability to drive and use machines
Dorzolamide has minor or moderate influence on the ability to drive and use machines.
Possible side effects such as dizziness and visual disturbances may affect the ability to drive and use machines (see also section 4.8).
4.8 Undesirable effects
Dorzolamide containing eye drops was evaluated in more than 1400 individuals in controlled and uncontrolled clinical studies. In long term studies of 1108 patients treated with Dorzolamide containing eye drops as monotherapy or as adjunctive therapy with an ophthalmic beta-blocker, the most frequent cause of discontinuation (approximately 3 %) from treatment with Dorzolamide containing eye drops was drug-related ocular adverse effects, primarily conjunctivitis and lid reactions.
The following adverse reactions have been reported either during clinical trials or during post-marketing experience:
Very common (> 1/10)
Common (> 1/100 to < 1/10)
Uncommon (> 1/1.000 to < 1/100)
Rare (> 1/10.000 to < 1/1,000)
Very rare (< 1/10.000)
Not known (cannot be estimated from the available data)
Nervous system and psychiatric disorders
Common: headache
Rare: dizziness, paraesthesia
Eye disorders
Very Common: burning and stinging
Common: superficial punctate keratitis, tearing, conjunctivitis, eyelid inflammation, eye itching, eyelid irritation, blurred vision
Uncommon: iridocyclitis
Rare: irritation including redness, pain, eyelid crusting, transient myopia (which resolved upon discontinuation of therapy), corneal oedema, ocular hypotony, choroidal detachment following filtration surgery
Respiratory, thoracic, and mediastinal disorders Rare: epistaxis
Gastrointestinal disorders Common: nausea, bitter taste Rare: throat irritation, dry mouth
Skin and subcutaneous tissue disorders
Rare: contact dermatitis, urticaria, pruritus, rash
Renal disorders Rare: urolithiasis
General disorders and administration site conditions Common: asthenia/fatigue
Rare: hypersensitivity: signs and symptoms of local reactions (palpebral reactions) and systemic allergic reactions including angioedema, shortness of breath, rarely bronchospasm
Laboratory findings
Dorzolamide was not associated with clinically meaningful electrolyte disturbances. Paediatric patients: see 5.1
4.9 Overdose
Only limited information is available with regard to human overdosage by accidental or deliberate ingestion of dorzolamide hydrochloride. The following have been reported with oral ingestion: somnolence; topical application: nausea, dizziness, headache, fatigue, abnormal dreams, and dysphagia.
Treatment should be symptomatic and supportive. Electrolyte imbalance, development of an acidotic state, and possible central nervous system effects may occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: antiglaucoma preparations and miotics, carbonic anhydrase inhibitor
ATC code: S01E C03
Mechanism of action
Carbonic anhydrase (CA) is an enzyme found in many tissues of the body including the eye. In humans, carbonic anhydrase exists as a number of isoenzymes, the most
active being carbonic anhydrase II (CAII) found primarily in red blood cells (RBCs) but also in other tissues. Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor secretion. The result is a reduction in intra-ocular pressure (IOP).
Dorzolamide contains dorzolamide hydrochloride, a potent inhibitor of human carbonic anhydrase II. Following topical ocular administration, dorzolamide reduces elevated intra-ocular pressure, whether or not associated with glaucoma. Elevated intra-ocular pressure is a major risk factor in the pathogenesis of optic nerve damage and visual-field loss. Dorzolamide does not cause pupillary constriction and reduces intra-ocular pressure without side effects such as night blindness, accommodative spasm. Dorzolamide has minimal or no effect on pulse rate or blood pressure.
Topically applied beta-adrenergic blocking agents also reduce IOP by decreasing aqueous humor secretion but by a different mechanism of action. Studies have shown that when dorzolamide is added to a topical beta-blocker, additional reduction in IOP is observed; this finding is consistent with the reported additive effects of beta-blockers and oral carbonic anhydrase inhibitors.
Pharmacodynamic effects Clinical effects Adult Patients:
In patients with glaucoma or ocular hypertension, the efficacy of dorzolamide given t.i.d. as monotherapy (baseline IOP > 23 mmHg) or given b.i.d. as adjunctive therapy while receiving ophthalmic beta-blockers (baseline IOP > 22 mmHg) was demonstrated in large-scale clinical studies of up to one- year duration. The IOP-lowering effect of dorzolamide as monotherapy and as adjunctive therapy was demonstrated throughout the day and this effect was maintained during long-term administration. Efficacy during long-term monotherapy was similar to betaxolol and slightly less than timolol. When used as adjunctive therapy to ophthalmic beta-blockers, dorzolamide demonstrated additional IOP lowering similar to pilocarpine 2% q.i.d.
Paediatric Patients:
A three month, double-masked, active-treatment controlled, multicentre study was undertaken in 184 (122 for dorzolamide) paediatric patients from one week of age to < 6 years of age with glaucoma or elevated intraocular pressure (baseline IOP > 22 mmHg) to assess the safety of 'Dorzolamide' when administered topically t.i.d. (three times a day). Approximately half the patients in both treatment groups were diagnosed with congenital glaucoma; other common aetiologies were Sturge Weber syndrome, iridocorneal mesenchymal dysgenesis, aphakic patients. The distribution by age and treatments in the monotherapy phase was as follows:
|
Dorzolamide 2% |
Timolol | |
|
Age cohort < 2 |
n=56 |
Timolol GS* 0.25% n=27 |
|
years |
Age range: 1 to 23 |
Age range: 0.25 to 22 |
|
months |
months |
|
Age cohort > 2 - < |
n=66 |
Timolol 0.5% n=35 |
|
6 years |
Age range: 2 to 6 years |
Age range: 2 to 6 years |
* gel-forming solution
Across both age cohorts approximately 70 patients received treatment for at least 61 days and approximately 50 patients received 81-100 days of treatment.
If IOP was inadequately controlled on dorzolamide or timolol gel-forming solution monotherapy, a change was made to open-label therapy according to the following: 30 patients < 2 years were switched to concomitant therapy with timolol gel-forming solution 0.25% daily and dorzolamide 2% t.i.d.; 30 patients > 2 years were switched to 2% dorzolamide/0.5% timolol fixed combination b.i.d.
Overall, this study did not reveal additional safety concerns in paediatric patients.
In a clinical trial, approximately 20% of patients while on dorzolamide monotherapy were observed to experience drug related adverse affects, the majority of which were local, non-serious ocular effects such as ocular burning and stinging, injection and eye pain. A small percentage < 4% were observed to have corneal oedema or haze. Local reactions appeared similar in frequency to comparator.
In post marketing data, metabolic acidosis in the very young particularly with renal immaturity/impairment has been reported.
Efficacy results in paediatric patients suggest that the mean IOP decrease observed in the dorzolamide group was comparable to the mean IOP decrease observed in the timolol group even if a slight numeric disadvantage was observed for timolol.
Longer-term efficacy studies (> 12 weeks) are not available.
5.2 Pharmacokinetic properties
Unlike oral carbonic anhydrase inhibitors, topical administration of dorzolamide hydrochloride allows for the drug to exert its effects directly in the eye at substantially lower doses and therefore with less systemic exposure. In clinical trials, this resulted in a reduction in IOP without the acid-base disturbances or alterations in electrolytes characteristic of oral carbonic anhydrase inhibitors.
When topically applied, dorzolamide reaches the systemic circulation. To assess the potential for systemic carbonic anhydrase inhibition following topical administration, drug and metabolite concentrations in RBCs and plasma and carbonic anhydrase inhibition in RBCs were measured. Dorzolamide accumulates in RBCs during chronic dosing as a result of selective binding to CAII while extremely low concentrations of free drug in plasma are maintained. The parent drug forms a single N-desethyl metabolite that inhibits CAII less potently than the parent drug but also inhibits a less active isoenzyme (CAI). The metabolite also accumulates in RBCs where it binds primarily to CAI. Dorzolamide binds moderately to plasma proteins (approximately 33%). Dorzolamide is primarily excreted unchanged in the urine; the metabolite is also excreted in urine. After dosing ends, dorzolamide washes out of RBCs non linearly, resulting in a rapid decline of drug concentration initially, followed by a slower elimination phase with a half-life of about four months.
When dorzolamide was given orally to simulate the maximum systemic exposure after long-term topical ocular administration, steady state was reached within 13 weeks. At steady state, there was virtually no free drug or metabolite in plasma; CA inhibition in RBCs was less than that anticipated to be necessary for a pharmacological effect on renal function or respiration. Similar pharmacokinetic results were observed after chronic, topical administration of dorzolamide.
However, some elderly patients with renal impairment (estimated CrCl 30-60 ml/min) had higher metabolite concentrations in RBCs, but no meaningful differences in carbonic anhydrase inhibition, and no clinically significant systemic side effects were directly attributable to this finding.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential.
In clinical studies, patients did not develop signs of metabolic acidosis or serum electrolyte changes that are indicative of systemic CA inhibition. Therefore, it is not expected that the effects noted in animal studies would be observed in patients receiving therapeutic doses of dorzolamide.
In rabbits, malformations of the vertebral bodies were observed at maternotoxic doses associated with metabolic acidosis.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Hydroxyethylcellulose
Mannitol
Sodium citrate
Sodium hydroxide/hydrochloric acid (for pH adjustment) Benzalkonium chloride Water for injections
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
After first opening: 4 weeks
From a microbiological point of view, once opened, the product may be stored for a maximum of 28 days. Other in-use storage times are the responsibility of the user.
6.4 Special precautions for storage
Do not store above 30°C!
Keep the bottle in the outer carton, in order to protect from light.
6.5 Nature and contents of container
Pack sizes
1 x 5 ml, 3 x 5 ml and 6 x 5 ml eye drops LDPE bottle with LDPE dropper and HDPE cap.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements.
See section 4.2 for patient instructions.
7 MARKETING AUTHORISATION HOLDER
Sandoz Limited Frimley Business Park,
Frimley,
Camberley,
Surrey,
GU16 7SR.
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 04416/0757
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
15/02/2011
10 DATE OF REVISION OF THE TEXT
15/02/2011