Dorzolamide/Timolol 20 Mg/Ml + 5 Mg/Ml Eye Drops Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist . This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Dorzolamide/Timolol is and what it is used for
2. What do you need to know before you use Dorzolamide/Timolol
3. How to use Dorzolamide/Timolol
4. Possible side effects
5. How to store Dorzolamide/Timolol
6. Contents of the pack and other information
1. WHAT DORZOLAMIDE/TIMOLOL IS AND WHAT IT IS USED FOR
Dorzolamide/Timolol is a combination of two medicines: dorzolamide and timolol.
• Dorzolamide belongs to a group of medicines called “carbonic anhydrase inhibitors”.
• Timolol belongs to a group of medicines called “beta blockers. Dorzolamide/Timolol is prescribed to lower raised pressure within the eye in the treatment of glaucoma when beta-blocker eye drops used alone are not adequate.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE DORZOLAMIDE/TIMOLOL
Do not use Dorzolamide/Timolol
• if you are allergic to dorzolamide, timolol, beta-blockers or any of the ingredients of this solution.
• if you have now or have had in past a respiratory disease such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough)
• if you have severe kidney problems, or a prior history of kidney stones,
• if you have a disturbance in the pH (acid/alkali balance) of your blood,
• if you have a slow heart rate, disorders of heart rhythm (irregular heart beats) or heart failure.
If you think any of these apply to you, do not use Dorzolamide/Timolol until you have consulted your doctor.
Warnings and precautions
Before you use this medicine, tell your doctor if you have now or have had in the past:
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, hypotension, Prinzmetal's angina (chest pains while resting), circulatory problems, low blood pressure
• breathing problems, asthma or chronic obstructive pulmonary disease
• peripheral arterial disease such as Raynaud's disease or Raynaud's syndrome
• diabetes or hypoglycaemia (low blood sugar) as timolol may mask signs and symptoms of low blood sugar
• overactivity of the thyroid gland as timolol may mask signs and symptoms
Tell your doctor before you have an operation (even at the dentist) that you use Dorzolamide/Timolol as timolol may change effects of some medicines used during anaesthesia or there may be a sudden fall in blood-pressure associated with the anaesthetic.
Tell your doctor if you now have or have had liver problems, if you have muscle weakness or have been diagnosed as having myasthenia gravis.
If you develop conjunctivitis (redness and irritation of the eye[s]), swelling of the eye or eyelids, skin rash, or itching in and around the eye contact your doctor immediately. Such symptoms may be due to an allergic reaction or may be a side-effect of Dorzolamide/Timolol (See ‘Possible Side Effects’).
Tell your doctor if you develop an eye infection, receive an eye injury, have eye surgery, develop other reactions or worsening of symptoms.
Contact lens use
If you wear soft contact lenses, it is important that your lenses are removed before using your eye drops and not put back into your eyes until 15 minutes after using your eye drops as the preservative benzalkonium chloride may possibly discolour the contact lenses.
If you have a history of heart disease your doctor may wish to monitor your pulse rate and other signs of this disease while you are using Dorzolamide/Timolol.
Use in children
There is limited experience with Dorzolamide/Timolol in infants and children. Other medicines and Dorzolamide/Timolol
Dorzolamide/Timolol can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicines or medicines to treat diabetes. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. This is particularly important if any of the following apply to you:
• You are taking antihypertensive medicines which are used to lower high blood pressure or medicines to treat heart disease such as calcium channel blockers and beta-blockers, guanethidine or digoxin
• You are taking medicines to treat a disturbed or uneven heartbeat such as quinidine (used to treat heart conditions and some types of malaria) digoxin or amiodarone
• You are using another eye drop that contains a beta-blocker
• You are taking another carbonic anhydrase inhibitor such as acetazolamide. You may be taking this type of medicine by mouth, as eye drops, or by some other method
• You are taking monoamine oxidase inhibitors (MAOIs) or selective serotonin reuptake inhibitors (SSRIs) both of which are used to treat depression or another illness
• You are taking a parasympathomimetic medicine which may have been prescribed to help you pass urine. Parasympathomimetics are a particular type of medicine which are sometimes used to help restore normal movements through the bowel
• You are taking narcotics such as morphine used to treat moderate to severe pain or if you are taking large doses of aspirin. Although there is no evidence that dorzolamide hydrochloride interacts with aspirin, some other medicines which are related to dorzolamide hydrochloride and which are taken by mouth, have been known to interact with aspirin
• You are taking medicines to treat diabetes or high blood sugar
• You are taking epinephrine (adrenaline).
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine. You should not use Dorzolamide/Timolol during pregnancy unless your doctor considers it necessary. Tell your doctor if you are pregnant or planning to become pregnant.
If treatment with Dorzolamide/Timolol is required, breast-feeding is not recommended. Tell your doctor if you are breast-feeding or intend to breast-feed. Driving and using machines
Dorzolamide/Timolol may cause side effects such as blurred vision in some patients. Do not drive or use any tools or machines until the symptoms have cleared. Dorzolamide/Timolol contains the preservative benzalkonium chloride.
• Benzalkonium chloride may cause eye irritation
• Benzalkonium chloride is known to discolour soft contact lenses. Avoid contact with soft contact lenses. Remove contact lenses prior to application and wait 15 minutes before re-insertion.
3. HOW TO USE DORZOLAMIDE/TIMOLOL
Always use Dorzolamide/Timolol exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The appropriate dosage and duration of treatment will be established by your doctor.
The usual dose is one drop in the affected eye(s) two times a day, for example in the morning and in the evening.
If you use Dorzolamide/Timolol with another topical eye medicine, leave at least 10 minutes between putting in Dorzolamide/Timolol and the other medicine.
Do not change the dosage of the drug without consulting your doctor. If you must stop treatment, contact your doctor immediately.
Do not allow the tip of the container to touch your eye or areas around your eye. It may become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the container, keep the tip of the container away from contact with any surface. In order to secure correct dosage - the dropper tip should not be enlarged.

Instructions for use:
It is recommended that you wash your hands before putting in your eye drops. It may be easier to apply your eye drops in front of a mirror.
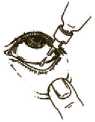
1. Before using the medication for the first time, be sure that the tamper-proof seal on the bottle neck is unbroken. A gap between the bottle and the cap is normal for an unopened bottle.
2. Take off the cap of the bottle.
3. Tilt your head back and gently pull your lower eyelid down to form a small pocket between your eyelid and your eye.

4. Invert the bottle, and squeeze it until a single drop is dispensed into the eye as directed by your doctor. DO NOT TOUCH YOUR EYE OR EYELID WITH THE DROPPER TIP.
5. After using Dorzolamide/Timolol, press a finger into the corner of your eye, by the nose for 2 minutes. This helps to stop timolol getting into the rest of the body.
6. If you use drops in both eyes, repeat the steps for your other eye.
7. Put the cap back on and close the bottle straight after you have used it.
If you use more Dorzolamide/Timolol than you should
It is important to keep to the dose your doctor has prescribed. If you put too many drops in your eye or swallow any of the contents of the bottle, you may feel unwell, for example you may become light-headed, have difficulty breathing, or feel that your heart rate has slowed. If you feel any of the above effects you should seek medical attention immediately.
If you forget to use Dorzolamide/Timolol
It is important to use Dorzolamide/Timolol as prescribed by your doctor.
If you miss a dose, apply it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to your regular dosing schedule.
Do not use a double dose to make up for forgotten individual doses.
If you stop taking Dorzolamide/Timolol
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Dorzolamide/Timolol can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Dorzolamide/Timolol without speaking to your doctor.
Like other topically applied ophthalmic drugs, dorzolamide and timolol (active substances of the medicinal product) are absorbed into the blood. This may cause similar side effects as seen with systemic beta-blocking agents and carbonic anhydrase inhibitors. The incidence of side effects after topical ophthalmic administration is lower than for systemic administration.
Listed adverse reactions include reactions seen within the class of ophthalmic beta-blockers and carbonic anhydrase inhibitors:
Very common (may affect more than 1 in 10 people): eye burning and stinging, distortion of the sense of taste.
Common (may affect up to 1 in 10 people): headache, eyelid or eye inflammation or irritation (itching, tearing, redness), dry eyes, conjunctival infection, blurred vision, corneal erosion, nausea, physical and/or mental weakness, inflammation of the paranasal sinuses (sinusitis).
Uncommon (may affect up to 1 in 100 people): inflammation of uvea (a part of the eyeball), depression, dizziness, loss of consciousness (syncope), visual disturbances, slow heart rate, shortness of breath, upset stomach, kidney stones. Rare (may affect up to 1 in 1,000 people): tingling, eye pain, eyelid crusting, corneal swelling, low intraocular pressure, drooping of the eyelid, double vision, ringing in ears, nosebleed, throat irritation, dry mouth, diarrhoea, skin rash, loss of hair, exacerbation of psoriasis, allergic reactions (swelling, urticaria, rash, itching, acute serious allergic reaction (anaphylactic reaction), sleeplessness, nightmares, memory loss, increase of signs and symptoms of myasthenia gravis (autoimmune neuromuscular disease leading to fluctuating muscle weakness), cerebrovascular accident, restriction in blood supply in brain, chest pain, strong heartbeat, swelling, irregular heartbeat, heart failure or arrest, low blood pressure, discolouration/coldness of the fingers and toes (Raynaud’s phenomenon) and coldness of the hands and feet, limping, sudden difficulty to breathe (bronchospasm), cough, respiratory failure, stuffy nose, systemic autoimmune disease (systemic lupus erythematosus), change of penis shape (Peyronie's disease), decreased libido.
Not known (frequency cannot be estimated from the available data): low blood sugar, abdominal pain, vomiting, pain of muscles, sexual dysfunction.
If any of the side effects get serious, or if you notice any side effects not mentioned in this leaflet, please, tell your doctor or pharmacist
5. HOW TO STORE DORZOLAMIDE/TIMOLOL
Keep this medicine out of the sight and reach of children.
Do not use Dorzolamide/Timolol after the expiry date which is stated on the bottle label and the carton after EXP:. The expiry date refers to the last day of that month.
This medicinal product does not require any special temperature storage conditions.
Dorzolamide/Timolol should be used within 28 days after the bottle is first opened. Therefore, you must throw away the bottle 4 weeks after you first opened it, even if some solution is left. To help you remember, write down the date that you opened it in the space on the carton.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Dorzolamide/Timolol contains
- The active substances are Dorzolamide and Timolol. Each ml contains 20mg dorzolamide (as 22.26mg of dorzolamide hydrochloride) and 5mg timolol (as 6.83mg of timolol maleate).
- The other ingredients are Mannitol (E421), Hydroxy Ethyl Cellulose, Benzalkonium Chloride (as a preservative), Sodium Citrate (E331), Sodium Hydroxide (E524) for pH adjustment and Water for injection.
What Dorzolamide/Timolol looks like and contents of the pack
Your medicine is in the form of a sterile, clear, slightly viscous, colourless aqueous eye drop solution.
Dorzolamide/Timolol is presented in a white opaque medium density polyethylene bottle with a sealed low density polyethylene dropper tip and a high density polyethylene cap with tamper proof seal, containing 5ml of the ophthalmic solution.
Pack size: 1, 3 or 6 bottles of 5ml each.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer The marketing authorisation holder is:
Zentiva, One Onslow Street, Guildford, Surrey, GU1 4YS, UK.
The manufacturer is:
Pharmathen S.A., 6 Dervenakion str., 15351 Pallini, Attiki, Greece or
Famar S.A., Plant A, 63 Agiou Dimitriou Street, 174 56 Alimos, Greece This leaflet was last revised in January 2012.
00000