Dymista Nasal Spray 137 Micrograms / 50 Micrograms Per Actuation Nasal Spray Suspension
Out of date information, search anotherneDO
0U/SO-OO98UOO17
56UK2071112-02-UP VCSIT
uojsuedsns 'AdjcI$ idsdn uoijonpo jed suudjBojoiuu 09 / suudjBojoiuu /£[
Aojds idsdn
Dymista®
3D|SjUiAG
Nasal Spray
137 micrograms / 50 micrograms per actuation Nasal Spray, Suspension
1. What Dymista Nasal Spray is and what it is used for
Package leaflet: Information for the patient
Dymista® Nasal Spray
137 micrograms/50 micrograms per actuation Nasal Spray, Suspension
Azelastine hydrochloride/fluticasone propionate
Read all of this leaflet carefully before you start
using this medicine because it contains important
information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Dymista Nasal Spray is and what it is used for
2. What you need to know before you use Dymista Nasal Spray
3. How to use Dymista Nasal Spray
4. Possible side effects
5. How to store Dymista Nasal Spray
6. Contents of the pack and other information
Dymista Nasal Spray contains two active substances: azelastine hydrochloride and fluticasone propionate.
• Azelastine hydrochloride belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of substances that the body produces as part of an allergic reaction -thus reducing symptoms of allergic rhinitis.
• Fluticasone propionate belongs to a group of medicines called corticosteroids which reduces inflammation.
Dymista Nasal Spray is used to relieve the symptoms of moderate to severe seasonal and perennial allergic rhinitis, for example: runny nose, post nasal drip, sneezing and itchy or blocked nose, if the use of either intranasal antihistamine or corticosteroid alone is not considered sufficient.
Seasonal and perennial allergic rhinitis are allergic reactions to substances such as pollen (hayfever), house mites, moulds, dust or pets.
2. What you need to know before you use Dymista Nasal Spray
Do not use Dymista Nasal Spray:
• If you are allergic to azelastine hydrochloride or fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Dymista Nasal Spray if:
• You had a recent operation on your nose.
• You have an infection in your nose. Infections of the nasal airways should be treated with antibacterial or antifungal medication. If you are given medication for an infection in your nose you can continue to use Dymista Nasal Spray to treat your allergies.
• You have tuberculosis or an untreated infection.
• You have a change in vision or a history of increased ocular pressure, glaucoma and/or cataracts. If this applies to you, you will be closely monitored whilst using Dymista Nasal Spray.
• You have problems with your adrenal glands. Care must be taken when transferring from systemic steroid treatment to Dymista Nasal Spray.
• You suffer from a severe liver disease. Your risk of suffering from systemic side effects is increased.
In these cases your doctor will decide whether you can use Dymista Nasal Spray.
Taking fluticasone may, when taken for a long time, cause children and adolescents to grow more slowly. The doctor will check your child's height regularly, and make sure he or she is taking the lowest possible effective dose.
If you are unsure whether the above applies to you, talk to your doctor or pharmacist before using Dymista Nasal Spray.
Children
This medicine is not recommended for children under 12 years.
Other medicines and Dymista Nasal Spray
Tell your doctor or pharmacist, if you are taking, have recently taken or might take any other medicines.
Please talk to your doctor or pharmacist:
• If you are taking medicines to treat HIV such as Ritonavir
• If you are taking medicines to treat fungal infections such as Ketoconazole.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using Dymista Nasal Spray.
Driving and using machines
Very rarely, you may experience fatigue or dizziness due to the disease itself or when using Dymista Nasal Spray. If this happens to you do not drive or operate machinery. Please be aware that drinking alcohol may enhance these effects.
Dymista Nasal Spray contains benzalkonium chloride
It may cause irritation of the nasal mucosa and bronchospasm. Tell your doctor or pharmacist if you feel discomfort when using the spray.
3. How to use Dymista Nasal Spray
Always use Dymista Nasal Spray exactly as your doctor has told you. Check with your doctor or pharmacist, if you are not sure.
It is important to use Dymista Nasal Spray regularly to gain full benefit from this treatment.
Contact with the eyes should be avoided.
Adults and adolescents (12 years and above)
• The recommended dose is one spray into each nostril in the morning and evening.
Use in children under 12 years
• This medicine is not recommended for children under 12 years.
Method of administration
For nasal use.
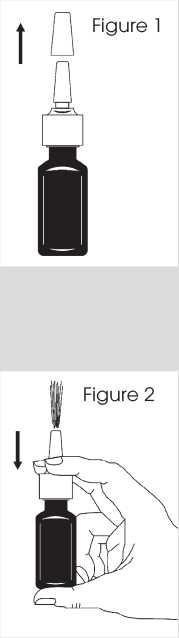
Figure 3

Figure 4

Read the following instructions carefully and use only as directed.
INSTRUCTION FOR USE
Preparing the spray
1. Shake the bottle gently for 5 seconds by tilting it upwards and downwards and then remove the protective cap (see figure 1).
2. The first time the nasal spray is used, you must prime the pump by squirting it into the air.
3. Prime the pump by putting two fingers on either side of the spray pump and place your thumb on the bottom of the bottle.
4. Press down and release the pump 6 times until a fine mist appears (see figure 2).
5. Now your pump is primed and ready to use.
6. If the nasal spray has not been used for more than 7 days, you will need to re-prime the pump once by pressing down and releasing the pump.
Using the spray
1. Blow your nose to clear your nostrils.
2. Keep your head tilted downwards towards your toes. Do not tilt head backwards.
3. Hold the bottle upright and carefully insert the spray tip into one nostril.
4. Close other nostril with your finger, rapidly press down once and sniff gently at the same time (see figure 3).
5. Breathe out through your mouth.
6. Repeat in your other nostril.
7. Breathe in gently, and do not tilt your head back after dosing. This will stop the medicine going into your throat and causing an unpleasant taste (see figure 4).
8. After each use wipe the spray tip with a clean tissue or cloth and then replace the protective cap.
It is important that you take your dose as advised by your doctor. You should use only as much as your doctor recommends.
Duration of Treatment
Dymista Nasal Spray is suitable for long-term use. Use this medicine for as long as your doctor tells you to.
If you use more Dymista Nasal Spray than you should
It is important that you take your dose as stated above or as advised by your doctor.
If you spray too much of this medicine into your nose you are unlikely to have any problems. However, treatment with higher than recommended doses of nasal corticosteroids may result in adrenal suppression, a condition that may produce weight loss, fatigue, muscle weakness, low blood sugar, salt cravings, joint pains, depression and darkening of the skin. If you are worried, contact your doctor.
If anyone, especially a child, accidentally drinks Dymista Nasal Spray, contact your doctor or nearest hospital casualty department as soon as possible.
If you forget to use Dymista Nasal Spray
Use your nasal spray as soon as you remember, then take the next dose at the usual time. Do not take a double dose to make up for a forgotten dose.
If you stop using Dymista Nasal Spray
Do not stop using Dymista Nasal Spray without asking your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines Dymista Nasal Spray can cause side effects, although not everybody gets them.
Stop taking Dymista and seek immediate medical help if you have any of the following symptoms:
• Swelling of face, lips, tongue or throat which may cause difficulty in swallowing/breathing and a sudden onset of skin rash. This could be signs of a severe allergic reaction. Please note: This is very rare.
• Cataract, glaucoma or increased pressure in your eye where you may have a loss of vision and/or red and painful eyes. These side effects are very rare.
Very common side effects
(These may affect more than 1 in 10 people):
• Nosebleed
Common side effects
(These may affect up to 1 in 10 people):
• Headache
• A bitter taste in your mouth, especially if you tilt your head backwards when you are using the nasal spray. This should go away if you have a soft drink a few minutes offer using this medicine.
• Unpleasant smell
Uncommon side effects
(These may affect up to 1 in 100 people):
• Slight irritation of the inside of the nose. This can cause mild stinging, itching or sneezing.
• Nasal dryness, cough, dry throat or throat irritation
6. Contents of the pack and other information
Rare side effects
(These may affect up to 1 in 1,000 people):
• Dry mouth
Very rare side effects
(These may affect up to 1 in 10,000 people):
• Dizziness or drowsiness
• Damage of the skin and mucous membrane in the nose
• Feeling sick, weary, exhausted or weak
• Rash, itchy skin or red, raised itchy bumps
• Bronchospasm (the narrowing of the airways in the lungs)
Side effects concerning the whole body may occur when this medicine is used at high doses for a long time.
In rare cases a reduction of bone density (osteoporosis) was observed when fluticasone was administered long-term.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at www.mhra.gov.uk/ yellowcard. Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy.
5. How to store Dymista Nasal Spray
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle label and the outer carton after "EXP". The expiry date refers to the last day of that month.
Do not refrigerate or freeze.
What Dymista Nasal Spray contains
The active substances are: azelastine hydrochloride and fluticasone propionate.
Each g of suspension contains 1000 micrograms azelastine hydrochloride and 365 micrograms fluticasone proprionate.
Each actuation (0.14 g) delivers 137 micrograms azelastine hydrochloride (= 125 micrograms azelastine) and 50 micrograms fluticasone propionate.
The other ingredients are: Disodium edetate, glycerol, microcrystalline cellulose, carmellose sodium, poly-sorbate 80, benzalkonium chloride solution, phenyl-ethyl alcohol and purified water.
What Dymista Nasal Spray looks like and contents of the pack
Dymista Nasal Spray is a white, homogenous suspension.
Dymista Nasal Spray comes in an amber coloured glass bottle fitted with a spray pump, applicator and a protective cap.
The 10 ml bottle contains 6.4 g nasal spray, suspension (at least 28 actuations). The 25 ml bottle contains 23 g nasal spray, suspension (at least 120 actuations).
Dymista Nasal Spray is presented in:
Packs containing 1 bottle with 6.4 g nasal spray, suspension
Packs containing 1 bottle with 23 g nasal spray, suspension
Multipacks comprising 10 bottles, each containing 6.4 g nasal spray, suspension
Multipacks comprising 3 bottles, each containing 23 g nasal spray, suspension.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Meda Pharmaceuticals Ltd Skyway Flouse Parsonage Road Takeley
Bishops Stortford CM22 6PU
Manufacturer
MEDA Pharma GmbFI & Co. KG Benzstr. 1, 61352 Bad Flomburg Germany
This leaflet was last revised in January 2013.
Shelf life offer first opening: Dispose of any unused medicine 6 months after you first open the nasal spray.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.