Dysport Injection 500 Units
o
o
o

ysport
Clostridium botulinum type A toxin-haemagglutinin complex
®
Taking other medicines:
Please tell your doctor if you are taking any antibiotics for an infection (e.g. aminoglycosides such as gentamicin or amikacin) or muscle relaxant drugs. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and Breast-feeding
If you are pregnant or breast-feeding you should ask your doctor for advice before taking any medicine. Dysport should not normally be used.
Use in children
Dysport should not be used in children younger than 2 years of age.
Driving and using machines
Dysport may cause muscle weakness or problems with your vision.
In this leaflet:
1. What Dysport is and what it is used for
2. Before you use Dysport
3. How Dysport is given
4. Possible side effects
5. How to store Dysport
6. Further information
1. WHAT DYSPORT IS AND WHAT IT IS USED FOR
Dysport is a toxin produced by Clostridium botulinum bacteria. The toxin works by stopping muscles contracting. It does this by preventing the release of a chemical in between the nerves and muscles which would normally make the muscles contract. This helps to reduce some of the abnormal muscle contractions known as spasms.
Dysport is used in adults to treat muscle spasms
• around the eyes
• in the face
• in the neck and shoulders
• in the arm
Dysport is also used to treat spasms in legs of children (aged two years or older) with cerebral palsy, to improve their walking.
You may also have physiotherapy
2. BEFORE YOU USE DYSPORT
Do not use Dysport:
If you are allergic to botulinum toxin or any of the ingredients (See section 6. for a list of ingredients).
Take special care with Dysport
There are increased risks of having Dysport injections under any of these circumstances.
Tell your doctor if:
• you have problems swallowing.
• you have any history of bronchitis, pneumonia or problems with breathing
• you have had an allergic reaction to a botulinum toxin in the past
• you have other problems or diseases that affect your muscles e.g. myasthenia gravis
• you bleed easily
• you have an infection where the injection will be given or if that area is swollen
Important information about one of the ingredients of Dysport
Dysport contains a small amount of albumin which has been obtained from human blood. The risk of passing on infections from blood cannot be eliminated completely when using human blood or products made from human blood.
If you experience any of these effects, do not drive or use any machines.
3. HOW DYSPORT IS GIVEN
Your doctor will give you your injection and decide how often you need treatment. This will depend on what you are being treated for.
A vial of Dysport should be used only for you and only for a single treatment session.
For treatment of muscle spasms in your arm:
The dose of Dysport will normally be 1000 units. The doctor may divide the amount between the affected arm muscles. Your muscle spasms should normally improve within 2 weeks.
For treatment of muscle spasms in your neck and shoulder.
The first dose of Dysport will normally be 500 units. The doctor will divide this amount into a number of places in the neck, probably into 2 or 3 of the neck muscles most affected by the condition. A smaller amount may be given to very underweight or elderly patients. Your muscle spasms should improve within 1 week. Further injections (250-1000 units) will be given about every 12 weeks depending on how long the effect lasts.
For treatment of muscle spasm around your eyes:
The first injection may be about 120 units per eye.
The medicine will be injected just under the skin at various sites around the eye. If only one eye is affected the doctor will only give injections of Dysport around this eye. Injections will be given about every 12 weeks depending on how long the effects last. On the next visits the amount of Dysport given may be reduced to 80 or 60 units per eye.
For treatment of muscle spasm in your face
The doctor will give you injections on the side of your face that is affected. The first injection may be about 120 units. Injections will be given about every 12 weeks depending on how long the effects last. Your next injections of Dysport may be reduced to 80 or 60 units.
For treatment of a child with cerebral palsy with muscle spasms in their legs.
The first dose of Dysport will be 20 units for each kg of the child's body weight. The doctor will divide the amount between both lower leg muscles. If only one leg is affected by spasms, the doctor will only give injections of 10 units per kg in this leg. Injections will be given about every 12 to 16 weeks. The dose your doctor gives the child could change depending on how they respond. The maximum dose is 1000 Units per patient each time.
If you are given more Dysport than you should
If you are given more Dysport than you need muscles other than the ones that were injected may begin to feel weak. This may not happen straightaway. If this happens, speak to your doctor immediately. Seek urgent medical help if you have difficulty breathing, swallowing or speaking.
Read all of this leaflet carefully before you start
using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have further questions, ask your doctor.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor.
O
J3
§
O
o

Clostridium botulinum type A toxin-haemagglutinin complex
®
|
Active Constituent Clostridium botulinum type A toxin- |
Per Vial |
|
haemagglutinin complex Other Constituents |
500U * |
|
Albumin solution |
125 MCG |
|
Lactose |
2.5 MG |
* One unit (U) is defined as the median lethal intraperitoneal dose in mice.
3 PHARMACEUTICAL FORM
Injection.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Dysport is indicated for focal spasticity, including the treatment of:
• arm symptoms associated with focal spasticity in conjunction with physiotherapy; and
• dynamic equinus foot deformity due to spasticity in ambulant paediatric cerebral palsy patients, two years of age or older, only in hospital specialist centres with appropriately trained personnel.
Dysport is also indicated for the following treatments:
• Spasmodic torticollis in adults
• Blepharospasm in adults
• Hemifacial spasm in adults
4.2 Posology and method of administration The units of Dysport are specific to the preparation and are not interchangeable with other preparations of botulinum toxin.
Training: Dysport should only be administered by appropriately trained physicians.
Ipsen can facilitate training in administration of Dysport injections.
The exposed central portion of the rubber stopper should be cleaned with alcohol immediately prior to piercing the septum. A sterile 23 or 25 gauge needle should be used.
Arm spasticity:
Posology
Adults. The recommended dose is 1000 units in total, distributed amongst the following five muscles:
|
Biceps brachii (BB) |
Flexor digitorum profundus (FDP) |
Flexor digitorum superficialis (FDS) |
Flexor carpi ulnaris (FCU) |
Flexor carpi radialis (FCR) |
Total Dose |
|
300-400 units (0.6-0.8 ml) |
150 units (0.3 ml) |
150-250 units (0.3-0.5 ml) |
150 units (0.3 ml) |
150 units (0.3 ml) |
1,000 units (2.0 ml) |
The sites of injection should be guided by standard locations used for electromyography, although actual location of the injection site will be determined by palpation. All muscles except the biceps brachii (BB) should be injected at one site, whilst the biceps should be injected at two sites.
The dose should be lowered if there is evidence to suggest that this dose may result in excessive weakness of the target muscles, such as for patients whose target muscles are small, where the BB muscle is not to be injected or patients who are to be administered multi-level injections. Clinical improvement may be expected within two weeks after injection. Data on repeated and long term treatment are limited.
Children: The safety and effectiveness of Dysport in the treatment of arm spasticity in children have not been demonstrated.
Method of administration
When treating arm spasticity, Dysport is reconstituted with 1.0ml of sodium chloride injection B.P (0.9%) to yield a solution containing 500 units per ml of botulinum toxin type A. Dysport is administered by intramuscular injection into the five muscles detailed above when treating arm spasticity.
Paediatric cerebral palsy spasticity:
Posology
The initial recommended dose is 20 units/kg body weight given as a divided dose between both calf muscles. If only one calf is affected, a dose of 10 units/kg bodyweight should be used. Consideration should be given to lowering this starting dose if there is evidence to suggest that this dose may result in excessive weakness of the target muscles, such as for patients whose target muscles are small or patients who require concomitant injections to other muscle groups. Following evaluation of response to the starting dose subsequent treatment may be titrated within the range 10 units/kg and 30 units/kg divided between both legs. The maximum dose administered must not exceed 1000 units/patient.
Administration should primarily be targeted to the gastrocnemius, although injections of the soleus and injection of the tibialis posterior should also be considered. The use of electromyography (EMG) is not routine clinical practice but may assist in identifying the most active muscles. Clinical improvement may be expected within two weeks after injection. Injections may be repeated approximately every 16 weeks or as required to maintain response, but not more frequently than every 12 weeks.
Method of administration
When treating paediatric cerebral palsy spasticity, Dysport is reconstituted with 1.0 ml of sodium chloride injection B.P (0.9%) to yield a solution containing 500 units per ml of botulinum toxin type A.
Dysport is administered by intramuscular injection into the calf muscles when treating spasticity.
Spasmodic torticollis Posology
Adults and elderly. The doses recommended for torticollis are applicable to adults of all ages providing the adults are of normal weight with no evidence of low neck muscle mass. A reduced dose may be appropriate if the patient is markedly underweight or in the elderly, where reduced muscle mass may exist.
The initial recommended dose for the treatment of spasmodic torticollis is 500 units per patient given as a divided dose and administered to the two or three most active neck muscles.
• For rotational torticollis distribute the 500 units by administering 350 units into the splenius capitis muscle, ipsilateral to the direction of the chin/head rotation and 150 units into the sternomastoid muscle, contralateral to the rotation.
• For laterocollis, distribute the 500 units by administering 350 units into the ipsilateral splenius capitis muscle and 150 units into the ipsilateral sternomastoid muscle. In cases associated with shoulder elevation the ipsilateral trapezoid or levator scapulae muscles may also require treatment, according to visible hypertrophy of the muscle or electromyographic (EMG) findings. Where injections of three muscles are required, distribute the 500 units as follows, 300 units splenius capitis, 100 units sternomastoid and 100 units to the third muscle.
• For retrocollis distribute the 500 units by administering 250 units into each of the splenius capitis muscles. This may be followed by bilateral trapezius injections (up to 250 units per muscle) after 6 weeks, if there is insufficient response. Bilateral splenii injections may increase the risk of neck muscle weakness.
• All other forms of torticollis are highly dependent on specialist knowledge and EMG to identify and treat the most active muscles. EMG should be used diagnostically for all complex forms of torticollis, for reassessment after unsuccessful injections in non complex cases, and for guiding injections into deep muscles or in overweight patients with poorly palpable neck muscles.
On subsequent administration, the doses may be adjusted according to the clinical response and side effects observed. Doses within the range of 250-1000 units are recommended, although the higher doses may be accompanied by an increase in side effects, particularly dysphagia. Doses above 1000 units are not recommended. The relief of symptoms of torticollis may be expected within a week after the injection. Injections should be repeated approximately every 12 weeks or as required to prevent recurrence of symptoms.
Children: The safety and effectiveness of Dysport in the treatment of spasmodic torticollis in children have not been demonstrated.
Method of administration
When treating spasmodic torticollis Dysport is reconstituted with 1.0 ml of sodium chloride injection B.P (0.9%) to yield a solution containing 500 units per ml of botulinum toxin type A. Dysport is administered by intramuscular injection as above when treating spasmodic torticollis.
Blepharospasm and hemifacial spasm Posology
Adults and elderly: In the treatment of bilateral blepharospasm the recommended initial dose is 120 units per eye.
Injection of 0.1 ml (20 units) should be made medially and of 0.2 ml (40 units) should be made laterally into the junction between the preseptal and orbital parts of both the upper and lower orbicularis oculi muscles of each eye.
For injections into the upper lid the needle should be directed away from its centre to avoid the levator muscle.
A diagram to aid placement of these injections is provided. The relief of symptoms may be expected to begin within two to four days with maximal effect within two weeks.
Injections should be repeated approximately every 12 weeks or as required to prevent recurrence of symptoms. On such subsequent administrations the dose may need to be reduced to 80 units per eye - viz -: 0.1 ml (20 units) medially and 0.1 ml (20 units) laterally above and below each eye in the manner previously described. The dose may be further reduced to 60 units per eye by omitting the medial lower lid injection.
0.1 ml
n O ml
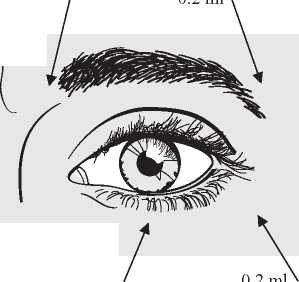
0.1 ml
0.2 ml
In cases of unilateral blepharospasm the injections should be confined to the affected eye. Patients with hemifacial spasm should be treated as for unilateral blepharospasm. The doses recommended are applicable to adults of all ages including the elderly.
Children: The safety and effectiveness of Dysport in the treatment of blepharospasm and hemifacial spasm in children have not been demonstrated.
Method of administration
When treating blepharospasm and hemifacial spasm Dysport is reconstituted with 2.5 ml of sodium chloride injection B.P (0.9%) to yield a solution containing 200 units per ml of botulinum toxin type A.
Dysport is administered by subcutaneous injection medially and laterally into the junction between the preseptal and orbital parts of both the upper and lower orbicularis oculi muscles of the eyes.
4.3 Contraindications
Dysport is contraindicated in individuals with known hypersensitivity to any components of Dysport.
4.4 Special warnings and precautions for use
Dysport should be administered with caution to patients with existing problems in swallowing or breathing as these problems can worsen following the distribution of the effect of toxin into the relevant muscles. Aspiration has occurred in rare cases and is a risk when treating patients who have a chronic respiratory disorder.
Patients with a history of dysphagia and aspiration should be treated with extreme caution.
Dysport should only be used with caution and under close supervision in patients with subclinical or clinical evidence of marked defective neuro-muscular transmission (e.g.myasthenia gravis). Such patients may have an increased sensitivity to agents such as Dysport which may result in excessive muscle weakness.
Patients treated with therapeutic doses may experience exaggerated muscle weakness. Patients with underlying neurological disorders including swallowing difficulties are at increased risk of these side effects. The botulinum toxin product should be used under specialist supervision in these patients and should only be used if the benefit of treatment is considered to outweigh the risk.
|
Dimensions: |
402 x 296 mm |
|
Specification ref: |
Leaflet-04 PS |
|
ICN: |
00548D |
|
Draft No.: |
THREE |
|
Artwork Amend Date: |
09/04/09 |
|
Colours: |
Black, P 361U |
|
Barcode type: |
N/A |
|
Front | |
Perforation:
3 mm x 1 mm parallel to 296 measurement
|
Component code: |
11010101XX |
|
Presentation: |
Dysport 500U |
|
Component: |
Pack insert |
|
Territory: |
UK |
|
Language: |
English |
|
Print supplier: |
M.Y. Newmarket |
Ipsen Biopharm Ltd,
Ash Road, Wrexham Ind. Est., Wrexham, LL13 9UF, UK.
Side effects related to spread of toxin distant from the site of administration have been reported (See section 4.8), which in some cases was associated with dysphagia, pneumonia and/or significant debility resulting in death very rarely.
Patients and their care-givers must be warned of the necessity of immediate medical treatment in case of problems with swallowing, speech or respiratory disorders. Careful consideration should be given before the injection of patients who have experienced a previous allergic reactions to a product containing botulinum toxin type A. The risk of a further allergic reaction must be considered in relation to the benefit of treatment.
Antibody formation to botulinum toxin has been noted rarely in patients receiving Dysport. Clinically, neutralizing antibodies have been detected by substantial deterioration in response to therapy and/or a need for consistently increasing doses.
For the treatment of cerebral palsy in children, Dysport should only be used in children over 2 years of age.
The recommended posology and frequency of administration for Dysport must not be exceeded (see section 4.2).
Dysport should only be used to treat a single patient, during a single session. Specific precautions must be taken for the preparation and administration of the product (see section 4.2) and for the inactivation and disposal of any unused reconstituted solution (see section 6.6).
As with any intramuscular injection, Dysport should be used only where strictly necessary in patients with prolonged bleeding times, infection or inflammation at the proposed injection site.
This product contains a small amount of human albumin.
The risk of transmission of viral infection cannot be excluded with absolute certainty following the use of human blood or blood products.
4.5 Interaction with other medicinal products and other forms of interaction
The effects of botulinum toxin may be enhanced by drugs interfering directly or indirectly with the neuromuscular function (e.g. aminoglycosides, curare-like non-depolarising blockers) and such drugs should be used with caution in patients treated with botulinum toxin.
4.6 Pregnancy and lactation
Teratological and other reproductive studies have not been performed with Dysport. The safety of its use in pregnant or lactating women has not been demonstrated.
Dysport should not be used in pregnant or lactating women, unless clearly necessary.
4.7 Effects on ability to drive and use machines
Dysport may impair the ability to drive or operate machinery in case of adverse reactions such as muscle weakness and eye disorders (diplopia, blurred vision, eyelid ptosis).
4.8 Undesirable effects
Very common >1/10: Common >1/100, <1/10:
Uncommon >1/1000, <1/100:
Rare >1/10 000, < 1/1000: Very rare <1/10 000.
Side effects related to spread of toxin distant from the site of administration have been reported (exaggerated muscle weakness, dysphagia, aspiration/aspiration pneumonia, with fatal outcome in some very rare cases).
(See section 4.4).
General
In the clinical trial programme, approximately 28% of the patients treated with Dysport experienced an adverse event.
The following adverse reactions were seen in patients treated across variety of indications including blepharospasm, hemifacial spasm, torticollis and spasticity associated with either cerebral palsy or stroke.
Nervous system disorders
Rare: Neuralgic amyotrophy
Skin and subcutaneous tissue disorders
Uncommon: Itching
Rare: Skin rashes
General disorders and administration site conditions
Common: Generalised weakness, fatigue, flu-like syndrome, pain / bruising at injection site.
In addition, the following adverse reactions specific to individual indications were reported.
Arm spasticity
Gastrointestinal disorders
Common: Dysphagia
Musculoskeletal and connective tissue disorders
Common: Arm muscle weakness
Injury, poisoning and procedural complications Common: Accidental injury/falls Paediatric cerebral palsy spasticity
Gastrointestinal disorders
Common: Diarrhoea, vomiting
Musculoskeletal and connective tissue disorders
Common: Leg muscle weakness
Renal and urinary disorders
Common: Urinary incontinence
General disorders and administration site conditions
Common: Abnormal gait
Injury, poisoning and procedural complications
Common: Accidental injury due to falling Accidental injury due to falling and abnormal gait may have been due to the over-weakening of the target muscle and/or the local spread of Dysport to other muscles involved in ambulation and balance.
Spasmodic torticollis
Nervous system disorders
Common: Dysphonia Uncommon: Headache Eye disorders
Uncommon: Diplopia, blurred vision Respiratory, thoracic and mediastinal disorders Rare: Respiratory disorders Gastrointestinal disorders
Very common: Dysphagia Uncommon: Dry mouth
Musculoskeletal and connective tissue disorders
Common: Neck muscle weakness
Dysphagia appeared to be dose related and occurred most frequently following injection into the sternomastoid muscle. A soft diet may be required until symptoms resolve. These side effects may be expected to resolve within two to four weeks.
Blepharospasm and hemifacial spasm Nervous system disorders
Common: Facial muscle weakness
Uncommon: Facial nerve paresis
Eye disorders
Very common: Ptosis
Common: Diplopia, dry eyes, tearing
Rare: Ophthalmoplegia
Skin and subcutaneous tissue disorders
Common: Eyelid oedema
Rare: Entropion
Side effects may occur due to deep or misplaced injections of Dysport temporarily paralysing other nearby muscle groups.
Post-marketing experience
The profile of adverse reactions reported to the company during post-marketing use reflects the pharmacology of the product and those seen during clinical trials. In addition, hypersensitivity reactions have been reported.
4.9 Overdose
Excessive doses may produce distant and profound neuromuscular paralysis. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. There is no specific antidote; antitoxin should not be expected to be beneficial and general supportive care is advised. Overdose could lead to an increased risk of the neurotoxin entering the bloodstream and may cause complications associated with the effects of oral botulinum poisoning (e.g deglutition and dysphonia). Symptomatic treatment should be instituted if necessary. In the event of an overdose the patient should be medically monitored for up to several weeks for symptoms of systemic weakness or muscle paralysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Clostridium botulinum type A toxin-haemagglutinin complex blocks peripheral cholinergic transmission at the neuromuscular junction by a presynaptic action at a site proximal to the release of acetylcholine. The toxin acts within the nerve ending to antagonise those events that are triggered by Ca2+ which culminate in transmitter release. It does not affect postganglionic cholinergic transmission or postganglionic sympathetic transmission.
The action of toxin involves an initial binding step whereby the toxin attaches rapidly and avidly to the presynaptic nerve membrane. Secondly, there is an internalisation step in which toxin crosses the presynaptic membrane, without causing onset of paralysis. Finally the toxin inhibits the release of acetylcholine by disrupting the Ca2+ mediated acetylcholine release mechanism, thereby diminishing the endplate potential and causing paralysis.
Recovery of impulse transmission occurs gradually as new nerve terminals sprout and contact is made with the post synaptic motor endplate, a process which takes 6 - 8 weeks in the experimental animal.
5.2 Pharmacokinetic properties Pharmacokinetic studies with botulinum toxin pose problems in animals because of the high potency, the minute doses involved, the large molecular weight of the compound and the difficulty of labelling toxin to produce sufficiently high specific activity. Studies using I125 labelled toxin have shown that the receptor binding is specific and saturable, and the high density of toxin receptors is a contributory factor to the high potency. Dose and time responses in monkeys showed that at low doses there was a delay of 2 - 3 days with peak effect seen 5 - 6 days after injection. The duration of action, measured by changes of ocular alignment and muscle paralysis varied between 2 weeks and 8 months. This pattern is also seen in man, and is attributed to the process of binding, internalisation and changes at the neuromuscular junction.
5.3 Preclinical safety data
There is no further pre-clinical information relevant to the prescribing physician that has not been included in other sections of the Summary of Product Characteristics.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Albumin and Lactose.
6.2 Incompatibilities None known.
6.3 Shelf life
The shelf life of the packaged product - 24 months at 2-8°C. The product may be stored for up to 8 hours at 2-8°C following reconstitution.
Since the product does not contain an anti-microbial agent, from a microbiological point of view, it is recommended that the product should be used immediately following reconstitution.
6.4 Special precautions for storage
Unopened vials must be maintained at temperatures between 2°C and 8°C. Dysport must be stored in a refrigerator at the hospital where the injections are to be carried out and should not be given to the patient to store. Reconstituted Dysport may be stored in a refrigerator (2-8°C) for up to 8 hours prior to use. Dysport should not be frozen.
6.5 Nature and contents of container Nature of container/closure:
Type 1 glass vials 3 ml capacity. 13 mm chlorbutyl freezedrying closures oversealed by 13 mm aluminium overseals with centre hole, crimped over.
Contents of container:
A white lyophilised powder for reconstitution.
6.6 Special precautions for disposal Immediately after treatment of the patient, any residual Dysport which may be present in either vial or syringe should be inactivated with dilute hypochlorite solution (1% available chlorine). Thereafter, all items should be disposed of in accordance with standard hospital practice.
Spillage of Dysport should be wiped up with an absorbent cloth soaked in dilute hypochlorite solution.
7 MARKETING AUTHORISATION HOLDER
Ipsen Limited, 190 Bath Road, Slough, Berkshire, SL1 3XE
8 MARKETING AUTHORISATION NUMBER(S)
PL 6958/0005
9 DATE OF FIRSTAUTHORISATION/RENEWAL OF THE AUTHORISATION
9th Dec 1990
10 DATE OF REVISION OF THE TEXT
March 2009
11 DOSIMETRY (IF APPLICABLE)
Not applicable.
12 INSTRUCTIONS FOR PREPARATION OF RADIOPHARMACEUTICALS
(IF APPLICABLE)
Not applicable.
D
m
2
O
m
J3
m
>
z
a
o
<
m
O
J3
5
o
I
m
If you forget an injection of Dysport
Nothing will happen if an injection is missed other than some of the spasm or muscle stiffness may return. Tell your doctor and he will decide when the next injection is needed.
If you stop taking Dysport
Your muscle movements will return to the way they were before treatment.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Dysport can cause side effects, although not everybody gets them.
Tell your doctor immediately if:
• you have any problems swallowing, breathing or with your speech
• you develop difficulty in breathing with or without swelling of the face, lips, tongue and /or throat, redness of the skin or an itchy lumpy rash (urticaria). This may mean you are having an allergic reaction to Dysport.
The chance of having a side effect is described by the following groups:
|
How often it occurs | |
|
Very Common |
Occurs in more than 1 in 10 patients treated |
|
Common |
in less than 1 in 10 patients treated |
|
Uncommon |
in less than 1 in 100 patients treated |
|
Rare |
in less than 1 in 1000 patients treated |
Some side effects may occur in any patient treated with Dysport whilst other side effects may depend on the condition treated. Make sure you read all the sections that apply to you.
Treatment of any condition (all patients)
Side effects that have occurred include:
Common:
• Bruising, or pain around the site where the injection was given or a burning sensation at the time the injection is given
• Generalised weakness
• Tiredness
• Flu-like symptoms Uncommon:
• Itching Rare:
• Skin rashes and muscle weakness
Other side effects related to the spread of Dysport away from the site of administration have also been reported (worsened muscle weakness, difficulty with swallowing or inhalation of foreign material which in very rare cases have been fatal).
Treatment of muscle spasms in the arm.
Side effects that have occurred include:
Common:
• Arm muscle weakness
• Accidental injury/falls
• Difficulty in swallowing
Treatment of muscle spasms in the eyes or face
Side effects that have occurred include:
Very common:
• Drooping eyelids Common:
• Dry eyes
• Double vision
• More tears than usual
• Swelling of the eyelid
• Facial muscle weakness Uncommon:
• Facial nerves may become paralysed
Rare:
• Difficulty in moving the eye
• The edge of the eyelid may turn in towards the eyeball and the eye muscles may become paralysed
Tell your doctor immediately if you notice very dry eyes.
Treatment of muscle spasms in the neck or shoulders.
Side effects that have occurred include:
Very common:
• Difficulty in swallowing. This side effect may be expected to resolve within 2 to 4 weeks.
Common:
• Neck muscle weakness
• A change to the tone of the voice Uncommon:
• Dry mouth
• Double or blurred vision
• Headache Rare:
• Dysport may cause breathing difficulties Treatment of children with muscle spasms in the leg
Side effects that have occurred include:
Common:
• The muscles of the lower leg may be weakened too much. This may change the way you walk or make you fall over more
• Urinary incontinence
• Diarrhoea
• Vomiting
If any of the side effects becomes serious or if you notice any side effects not listed in this leaflet, please tell your doctor.
5. HOW TO STORE DYSPORT
Keep out of the reach and sight of children.
Do not use after the expiry date shown on the box. Dysport will be stored in a refrigerator (2°C-8°C) at the place where the injections are carried out. This medicine should not be given to patients to store.
6. FURTHER INFORMATION
What Dysport contains
The active constituent of Dysport is Clostridium botulinum complex (500 units). Dysport also contains human albumin and lactose. Before it is injected Dysport will be dissolved in sodium chloride for injection (a solution of salt).
What Dysport looks like and contents of the pack
Dysport is a white powder in a glass container called a vial. Each pack contains 2 vials of Dysport
Marketing Authorisation Holder and manufacturer
The marketing authorization holder is:
Ipsen Limited, 190 Bath Road, Slough, Berkshire,
SL1 3XE, UK.
Dysport is manufactured by:
Ipsen Biopharm Limited, Ash Road, Wrexham Industrial Estate, Wrexham LL13 9Uf.
This leaflet was last approved in
February 2009
Product licence number
PL 06958/0005

IP5EIM
|
Dimensions: Specification ref: |
402 x 296 mm Leaflet-04 PS |
|
ICN: Draft No.: Artwork Amend Date: |
00548D THREE04 09/04/09 |
|
Colours: |
Black, P 361U |
|
Barcode type: |
N/A |
|
Back | |
|
Perforation: | |
|
See other side | |
|
Component code: |
11010101XX |
|
Presentation: |
Dysport 500U |
|
Component: |
Pack insert |
|
Territory: |
UK |
|
Language: |
English |
|
Print supplier: |
M.Y. Newmarket |
Ipsen Biopharm Ltd,
Ash Road, Wrexham Ind. Est., Wrexham, LL13 9UF, UK.
Tel: +44 (0) 1978 661181
Fax: +44 (0) 1978 667569