Elumatic Iii Technetium Generator
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
ELUMATIC III® TECHNETIUM [99mTc] GENERATOR
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The generator ELUMATIC III® is a system allowing elution of sodium pertechnetate [99mTc] injection (fission). The obtained technetium-99m solution is sterile and pyrogen-free, and complies with the requirements of the European Pharmacopoeia and of the U.S. Pharmacopoeia, including those for radiochemical purity (more than or equal to 95 %, average analysis : 99%), and radionuclidic purity (at calibration date : 99Mo < 0.1 %, 131I < 5.10-3 %, 103Ru < 5.10-3 %, 89Sr < 6.10"5 %, 90Sr < 6.10-6 %, alpha-emitting impurities < 1.10" %, other gamma-emitting impurities < 0.01 %). The solution is clear and colourless, with a pH ranging between 4.0 and 8.0, and contains no antimicrobial preservative. It is eluted from alumina chromatographic column on which fission produced molybdenum-99 (T1/2 = 66 h) parent of technetium-99m (T1/2 = 6.02 h) is fixed. The system is automatic and highly shielded.
Description
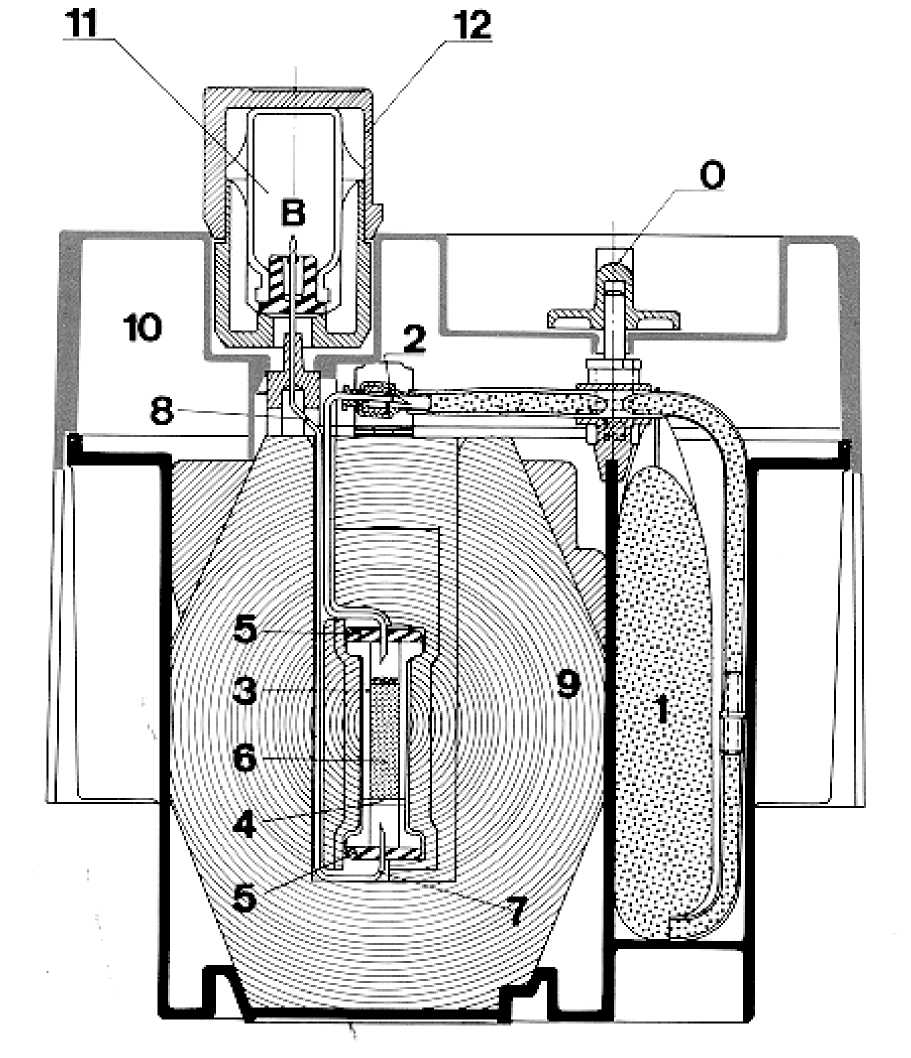
The system includes:
- A supple plastic bag (1) containing the eluent (0.9 % sodium chloride and 0.005 % sodium nitrate aqueous solution). The bag is connected by a stainless steel needle (2) to the top of the chromatographic column.
- A glass chromatographic column (3) with a filter at the bottom (4) to prevent any leakage of alumina. The column is obturated at both ends by caps maintained by metallic capsules (5). This column contains the alumina (6) which adsorbs the molybdate ions and is inert towards the pertechnetate ions.
- A needle (7) with one end connected to the bottom of the column. The other end (8) is to receive either a vacuum vial to elute the column, or a vial with a bacteriostatic solution so as to preserve sterility between two elutions.
The column and the needles are protected by a cylindro-conical lead shielding (9) with a minimal thickness of 52 mm. The whole system is placed in a parallelepipedic cover (23 x 21 x 14 cm) made of moulded nylon (10).
At the top part of the cover is the elution station protected by a (12) cylindrical container (B) in which a vial with a bacteriostatic solution (11) is placed. The end of the needle plunges in the solution. This vial contains an aqueous solution of lauryl bromide dimethylbenzylammonium (0.02 %).
Near the elution station is a cavity with a safety valve (0) turned off during the transport (O).
The sodium pertechnetate [99mTc] injection obtained meets the requirements of the European Pharmacopoeia and of the U.S. Pharmacopoeia.
The ELUMATIC III® is delivered in a tight metal drum.
Quantitative composition
Activity of the generator at the calibration date
As required 200 mL
Sodium pertechnetate [99mTc] (technetium-99m produced by radioactive decay of the parent radionuclide fission molybdenum-99 adsorbed on alumina Alumina
Elution solution containing 0.9 % sodium chloride and 0.005 % sodium nitrate in a PVC bag
0.25 mL per vial of bacteriostatic solution
Bacteriostatic solution (lauryl-dimethyl-benzylammonium bromide)
Available activities
2 4 6 8 10 12 16 20 GBq
54 108 162 216 270 324 432 540 mCi
They are expressed in available technetium-99m, not in molybdenum-99.
Physical characteristics
Technetium-99m is produced by means of radioactive decay of molybdenum-99. Technetium-99m decays with the emission of gamma radiation with a mean energy of 140 keV and a half-life of 6.02 hours to technetium-99 which, in view of its long half-life of 2.13 x 105 years can be regarded as quasi stable.
Decay table for 99Mo (half-life : 66 hours)
Days Hours %
|
Days Hours % |
Days Hours % |
Days Hours % |
Days Hours % |
|
-8 d |
-192 |
750.82 |
-4 d |
-96 |
274.01 |
Calib 0 |
100.00 |
4 d |
96 |
36.49 |
8 d |
192 |
13.31 |
12 d |
288 |
4.8 | |
|
-190 |
735.22 |
-94 |
268.32 |
ra- |
2 |
97.92 |
98 |
35.73 |
194 |
13.04 |
290 |
6 | |||||
|
-188 |
719.94 |
-92 |
262.74 |
tion |
4 |
95.89 |
100 |
34.99 |
196 |
12.77 |
292 |
4.7 | |||||
|
-186 |
704.96 |
-90 |
257.28 |
date |
6 |
93.89 |
102 |
34.26 |
198 |
12.50 |
294 |
6 | |||||
|
-184 |
690.33 |
-88 |
251.93 |
8 |
91.94 |
104 |
33.55 |
200 |
12.24 |
296 |
4.6 | ||||||
|
-182 |
675.98 |
-86 |
246.70 |
10 |
90.03 |
106 |
32.85 |
202 |
11.99 |
298 |
6 | ||||||
|
-180 |
661.94 |
-84 |
241.57 |
12 |
88.16 |
108 |
32.17 |
204 |
11.74 |
300 |
4.5 | ||||||
|
-178 |
648.18 |
-82 |
236.55 |
14 |
86.33 |
110 |
31.50 |
206 |
11.49 |
302 |
6 | ||||||
|
-176 |
634.71 |
-80 |
231.64 |
16 |
84.53 |
112 |
30.84 |
208 |
11.25 |
304 |
4.4 | ||||||
|
-174 |
621.52 |
-78 |
226.82 |
18 |
82.78 |
114 |
30.20 |
210 |
11.02 |
306 |
7 | ||||||
|
-172 |
608.61 |
-76 |
222.11 |
20 |
81.06 |
116 |
29.57 |
212 |
10.79 |
308 |
4.3 | ||||||
|
-170 |
595.96 |
-74 |
217.49 |
22 |
79.37 |
118 |
28.96 |
214 |
10.57 |
310 |
8 | ||||||
|
1 d |
4.2 | ||||||||||||||||
|
-7 d |
-168 |
583.57 |
-3 d |
-72 |
213.01 |
24 |
77.72 |
5 d |
120 |
28.36 |
9 d |
216 |
10.35 |
13 d |
312 |
9 | |
|
-166 |
571.45 |
-70 |
208.58 |
26 |
76.10 |
122 |
27.77 |
218 |
10.13 |
314 |
4.2 | ||||||
|
-164 |
559.57 |
-68 |
204.25 |
28 |
74.52 |
124 |
27.19 |
220 |
9.92 |
316 |
0 | ||||||
|
-162 |
547.94 |
-66 |
200.00 |
30 |
72.97 |
126 |
26.63 |
222 |
9.72 |
318 |
4.1 | ||||||
|
-160 |
536.56 |
-64 |
195.84 |
32 |
71.46 |
128 |
26.07 |
224 |
9.51 |
320 |
1 | ||||||
|
-158 |
525.41 |
-62 |
191.77 |
34 |
69.97 |
130 |
25.53 |
226 |
9.32 |
322 |
4.0 | ||||||
|
-156 |
514.49 |
-60 |
187.79 |
36 |
98.52 |
132 |
25.00 |
228 |
9.12 |
324 |
2 | ||||||
|
-154 |
503.80 |
-58 |
183.88 |
38 |
67.09 |
134 |
24.48 |
230 |
8.93 |
326 |
3.9 | ||||||
|
-152 |
493.33 |
-56 |
180.06 |
40 |
65.70 |
136 |
23.97 |
232 |
8.75 |
328 |
4 | ||||||
|
-150 |
483.07 |
-54 |
176.32 |
42 |
64.33 |
138 |
23.47 |
234 |
8.56 |
330 |
3.8 | ||||||
|
-148 |
473.04 |
-52 |
172.65 |
44 |
63.00 |
140 |
22.99 |
236 |
8.39 |
332 |
6 | ||||||
|
-146 |
463.21 |
-50 |
169.06 |
2 d |
46 |
61.69 |
142 |
22.51 |
238 |
8.21 |
334 |
3.7 | |||||
|
-6 d |
-144 |
453.58 |
-2 d |
-48 |
165.55 |
48 |
60.40 |
6 d |
144 |
22.04 |
10 d |
240 |
8.04 |
14 d |
336 |
8 | |
|
-142 |
444.15 |
-46 |
162.11 |
50 |
59.15 |
146 |
21.58 |
242 |
7.87 |
3.7 | |||||||
|
-140 |
434.92 |
-44 |
158.74 |
52 |
57.92 |
148 |
21.13 |
244 |
7.71 |
0 | |||||||
|
-138 |
425.89 |
-42 |
155.44 |
54 |
56.872 |
150 |
20.69 |
246 |
7.55 |
3.6 | |||||||
|
-136 |
417.04 |
-40 |
152.21 |
56 |
55.54 |
152 |
20.26 |
248 |
7.39 |
2 | |||||||
|
-134 |
408.37 |
-38 |
149.05 |
58 |
54.38 |
154 |
19.84 |
250 |
7.24 |
3.5 | |||||||
|
-132 |
399.88 |
-36 |
145.95 |
60 |
53.25 |
156 |
19.43 |
252 |
7.09 |
5 | |||||||
|
-130 |
391.57 |
-34 |
142.91 |
62 |
52.15 |
158 |
19.03 |
254 |
6.94 |
3.4 | |||||||
|
-128 |
383.44 |
-32 |
139.94 |
64 |
51.06 |
160 |
18.63 |
256 |
6.80 |
7 | |||||||
|
-126 |
375.47 |
-30 |
137.04 |
66 |
50.00 |
162 |
18.24 |
258 |
6.66 |
3.4 | |||||||
|
-124 |
367.66 |
-28 |
134.19 |
68 |
48.96 |
164 |
17.86 |
260 |
6.52 |
0 | |||||||
|
-122 |
360.02 |
-26 |
131.40 |
70 |
47.94 |
166 |
17.49 |
262 |
6.38 |
3.3 | |||||||
|
3 d |
3 | ||||||||||||||||
|
-5 d |
-120 |
352.54 |
-1 d |
-24 |
128.67 |
72 |
46.95 |
7 d |
168 |
17.13 |
11 d |
264 |
6.25 |
3.2 | |||
|
-118 |
345.22 |
-22 |
125.99 |
74 |
45.97 |
170 |
16.77 |
266 |
6.12 |
6 | |||||||
|
-116 |
338.04 |
-20 |
123.37 |
76 |
45.02 |
172 |
16.42 |
268 |
5.99 |
3.1 | |||||||
|
-114 |
331.02 |
-18 |
120.81 |
78 |
44.08 |
174 |
16.08 |
270 |
5.87 |
9 | |||||||
|
-112 |
324.14 |
-16 |
118.30 |
80 |
43.16 |
176 |
15.75 |
272 |
5.75 |
3.1 | |||||||
|
-110 |
317.40 |
-14 |
115.84 |
82 |
42.27 |
178 |
15.42 |
274 |
5.63 |
3 | |||||||
|
-108 |
310.81 |
-12 |
113.43 |
84 |
41.39 |
180 |
15.10 |
276 |
5.51 |
3.0 | |||||||
|
-106 |
304.35 |
-10 |
111.07 |
86 |
40.53 |
182 |
14.79 |
278 |
5.40 |
6 | |||||||
|
-104 |
298.02 |
-8 |
108.76 |
88 |
39.69 |
184 |
14.48 |
280 |
5.28 |
3.0 | |||||||
|
-102 |
291.83 |
-6 |
106.50 |
90 |
38.86 |
186 |
14.18 |
282 |
5.17 |
0 | |||||||
|
-100 |
285.77 |
-4 |
104.29 |
92 |
38.05 |
188 |
13.88 |
284 |
5.07 | ||||||||
|
-98 |
279.83 |
-2 |
102.12 |
94 |
37.26 |
190 |
13.60 |
286 |
4.96 |
2.9 4 | |||||||
TABLE 1
|
Decay table for |
99mTc ( |
ialf-life: 6.02 hours) : | |||||||||
|
H.Min% |
H.Min % |
H.Min % |
H.Min % |
H.Min % |
H.Min % | ||||||
|
0.05 |
99.05 |
2.05 |
78.67 |
4.05 |
62.49 |
6.05 |
49.64 |
8.05 |
39.43 |
10.05 |
31.32 |
|
0.10 |
98.10 |
2.10 |
77.92 |
4.10 |
61.89 |
6.10 |
49.16 |
8.10 |
39.05 |
10.10 |
31.02 |
|
0.15 |
97.16 |
2.15 |
77.18 |
4.15 |
61.30 |
6.15 |
48.69 |
8.15 |
38.68 |
10.15 |
30.72 |
|
0.20 |
96.23 |
2.20 |
76.44 |
4.20 |
60.72 |
6.20 |
48.23 |
8.20 |
38.31 |
10.20 |
30.43 |
|
0.25 |
95.32 |
2.25 |
75.71 |
4.25 |
60.14 |
6.25 |
47.77 |
8.25 |
37.94 |
10.25 |
30.14 |
|
0.30 |
94.41 |
2.30 |
74.99 |
4.30 |
59.56 |
6.30 |
47.31 |
8.30 |
37.58 |
10.30 |
29.85 |
|
0.35 |
93.50 |
2.35 |
74.27 |
4.35 |
58.99 |
6.35 |
46.86 |
8.35 |
37.22 |
10.35 |
29.57 |
|
0.40 |
92.61 |
2.40 |
73.56 |
4.40 |
58.43 |
6.40 |
46.41 |
8.40 |
36.87 |
10.40 |
29.28 |
|
0.45 |
91.73 |
2.45 |
72.86 |
4.45 |
57.87 |
6.45 |
45.97 |
8.45 |
36.51 |
10.45 |
29.00 |
|
0.50 |
90.85 |
2.50 |
72.16 |
4.50 |
57.32 |
6.50 |
45.53 |
8.50 |
36.17 |
10.50 |
28.73 |
|
0.55 |
89.98 |
2.55 |
71.47 |
4.55 |
56.77 |
6.55 |
45.10 |
8.55 |
35.82 |
10.55 |
28.45 |
|
1.00 |
89.12 |
3.00 |
70.79 |
5.00 |
56.23 |
7.00 |
44.66 |
9.00 |
35.48 |
11.00 |
28.18 |
|
1.05 |
88.27 |
3.05 |
70.12 |
5.05 |
55.69 |
7.05 |
44.24 |
9.05 |
35.14 |
11.05 |
27.91 |
|
1.10 |
87.43 |
3.10 |
69.45 |
5.10 |
55.16 |
7.10 |
43.82 |
9.10 |
34.80 |
11.10 |
27.64 |
|
1.15 |
86.60 |
3.15 |
68.78 |
5.15 |
54.64 |
7.15 |
43.40 |
9.15 |
34.47 |
11.15 |
27.38 |
|
1.20 |
85.77 |
3.20 |
68.13 |
5.20 |
54.11 |
7.20 |
42.98 |
9.20 |
34.14 |
11.20 |
27.12 |
|
1.25 |
84.95 |
3.25 |
67.48 |
5.25 |
53.60 |
7.25 |
42.57 |
9.25 |
33.82 |
11.25 |
26.86 |
|
1.30 |
84.14 |
3.30 |
66.83 |
5.30 |
53.09 |
7.30 |
42.17 |
9.30 |
33.49 |
11.30 |
26.60 |
|
1.35 |
83.33 |
3.35 |
66.19 |
5.35 |
52.58 |
7.35 |
41.76 |
9.35 |
33.17 |
11.35 |
26.35 |
|
1.40 |
82.54 |
3.40 |
66.56 |
5.40 |
52.08 |
7.40 |
41.36 |
9.40 |
32.86 |
11.40 |
26.10 |
|
1.45 |
81.75 |
3.45 |
64.94 |
5.45 |
51.58 |
7.45 |
40.97 |
9.45 |
32.54 |
11.45 |
25.85 |
|
1.50 |
80.97 |
3.50 |
64.32 |
5.50 |
51.09 |
7.50 |
40.58 |
9.50 |
32.23 |
11.50 |
25.60 |
|
1.55 |
80.20 |
3.55 |
63.70 |
5.55 |
50.60 |
7.55 |
40.19 |
9.55 |
31.92 |
11.55 |
25.36 |
|
2.00 |
79.43 |
4.00 |
63.09 |
6.00 |
50.12 |
8.00 |
39.81 |
10.00 |
31.62 |
12.00 |
25.12 |
TABLE 2
The maximal radioactivity of elutable sodium pertechnetate [99mTc] for each content of generator can be determined by reference to the following table:
|
-8 |
-7 |
-6 |
-5 |
-4 |
-3 |
-2 |
-1 |
0 |
+1 |
+2 |
+3 |
+4 |
+5 |
+6 |
+7 |
+8 |
+9 |
+10 |
+11 |
+12 |
+13 |
+14 | |||
|
GBq |
2 |
15. |
11. |
9.0 |
7.0 |
5.4 |
4.2 |
3.3 |
2.5 |
2 |
1.5 |
1.2 |
0.9 |
0.7 |
0.5 |
0.4 |
0.3 |
0.2 |
0.2 |
0.1 |
0.1 |
0.1 |
0.0 |
0.0 |
2 |
|
02 |
67 |
7 |
5 |
8 |
6 |
1 |
7 |
5 |
1 |
4 |
3 |
7 |
4 |
4 |
7 |
1 |
6 |
3 |
0 |
8 |
6 | ||||
|
mCi |
54 |
405 |
315 |
245 |
190 |
148 |
115 |
89 |
69 |
54 |
42 |
33 |
25 |
20 |
15 |
12 |
9 |
7 |
6 |
4 |
3 |
3 |
2 |
2 |
54 |
|
GBq |
4 |
30. |
23. |
18. |
14. |
10. |
8.5 |
6.6 |
5.1 |
4 |
3.1 |
2.4 |
1.8 |
1.4 |
1.1 |
0.8 |
0.6 |
0.5 |
0.4 |
0.3 |
0.2 |
0.1 |
0.1 |
0.1 |
4 |
|
03 |
34 |
14 |
10 |
96 |
2 |
2 |
5 |
1 |
2 |
8 |
6 |
3 |
8 |
9 |
3 |
1 |
2 |
5 |
9 |
5 |
2 | ||||
|
mCi |
108 |
811 |
630 |
490 |
381 |
296 |
230 |
179 |
139 |
108 |
84 |
65 |
51 |
39 |
31 |
24 |
19 |
14 |
11 |
9 |
7 |
5 |
4 |
3 |
108 |
|
GBq |
6 |
45. |
35. |
27. |
21. |
16. |
12. |
9.9 |
7.7 |
6 |
4.6 |
3.6 |
2.8 |
2.1 |
1.7 |
1.3 |
1.0 |
0.8 |
0.6 |
0.4 |
0.3 |
0.2 |
0.2 |
0.1 |
6 |
|
05 |
01 |
21 |
15 |
44 |
78 |
3 |
2 |
6 |
2 |
2 |
9 |
0 |
2 |
3 |
0 |
2 |
8 |
8 |
9 |
3 |
8 | ||||
|
mCi |
162 |
121 6 |
945 |
735 |
571 |
444 |
345 |
268 |
208 |
162 |
126 |
98 |
76 |
59 |
46 |
36 |
28 |
22 |
17 |
13 |
10 |
8 |
6 |
5 |
162 |
|
GBq |
8 |
60. |
46. |
36. |
28. |
21. |
17. |
13. |
10. |
8 |
6.2 |
4.8 |
3.7 |
2.9 |
2.2 |
1.7 |
1.3 |
1.0 |
0.8 |
0.6 |
0.5 |
0.3 |
0.3 |
0.2 |
8 |
|
07 |
69 |
29 |
20 |
92 |
04 |
24 |
29 |
2 |
3 |
6 |
2 |
7 |
6 |
7 |
7 |
3 |
4 |
0 |
9 |
0 |
4 | ||||
|
mCi |
216 |
162 2 |
126 1 |
980 |
761 |
592 |
460 |
358 |
278 |
216 |
168 |
130 |
101 |
79 |
61 |
48 |
37 |
29 |
22 |
17 |
14 |
10 |
8 |
6 |
216 |
|
GBq mCi |
10 270 |
75. 08 202 7 |
58. 36 157 6 |
45. 36 122 5 |
35. 25 952 |
27. 40 740 |
21. 30 575 |
16. 55 447 |
12. 87 347 |
10 270 |
7.7 7 210 |
6.0 4 163 |
4.7 0 127 |
3.6 5 99 |
2.8 4 77 |
2.2 0 60 |
1.7 1 46 |
1.3 3 36 |
1.0 4 28 |
0.8 0 22 |
0.6 3 17 |
0.4 9 13 |
0.3 8 10 |
0.2 9 8 |
10 270 |
|
GBq |
12 |
90. |
70. |
54. |
42. |
32. |
25. |
19. |
15. |
12 |
9.3 |
7.2 |
5.6 |
4.3 |
3.4 |
2.6 |
2.0 |
1.6 |
1.2 |
0.9 |
0.7 |
0.5 |
0.4 |
0.3 |
12 |
|
10 |
03 |
43 |
31 |
88 |
56 |
86 |
44 |
3 |
5 |
3 |
8 |
0 |
5 |
6 |
0 |
4 |
6 |
5 |
8 |
5 |
5 | ||||
|
mCi |
324 |
243 |
189 |
147 |
114 |
888 |
690 |
536 |
417 |
324 |
252 |
196 |
152 |
118 |
61 |
71 |
56 |
43 |
34 |
26 |
20 |
16 |
12 |
10 |
324 |
|
3 |
1 |
0 |
2 | ||||||||||||||||||||||
|
GBq |
16 |
120 |
93. |
72. |
56. |
43. |
34. |
26. |
20. |
16 |
12. |
9.6 |
7.5 |
5.8 |
4.5 |
3.5 |
2.7 |
2.1 |
1.6 |
1.2 |
1.0 |
0.7 |
0.6 |
0.4 |
16 |
|
.13 |
37 |
57 |
41 |
84 |
08 |
49 |
59 |
44 |
7 |
1 |
4 |
4 |
3 |
4 |
3 |
6 |
9 |
0 |
8 |
0 |
7 | ||||
|
mCi |
432 |
324 |
252 |
195 |
152 |
118 |
920 |
715 |
556 |
432 |
336 |
261 |
203 |
158 |
123 |
95 |
74 |
58 |
45 |
35 |
27 |
21 |
16 |
13 |
432 |
|
4 |
1 |
9 |
3 |
4 | |||||||||||||||||||||
|
GBq |
20 |
150 |
116 |
90. |
70. |
54. |
42. |
33. |
25. |
20 |
15. |
12. |
9.3 |
7.3 |
5.6 |
4.4 |
3.4 |
2.6 |
2.0 |
1.6 |
1.2 |
0.9 |
0.7 |
0.6 |
20 |
|
.16 |
.71 |
72 |
51 |
80 |
59 |
11 |
73 |
54 |
08 |
9 |
0 |
7 |
1 |
3 |
6 |
7 |
1 |
5 |
7 |
6 |
0 | ||||
|
mCi |
540 |
405 |
315 |
244 |
190 |
148 |
115 |
894 |
695 |
540 |
420 |
326 |
254 |
197 |
153 |
119 |
93 |
72 |
56 |
43 |
34 |
26 |
20 |
16 |
540 |
|
4 |
1 |
9 |
4 |
0 |
0 |
TABLE 3
Note: The days with a minus sign are the days preceding the date shown on the label (calibration date) and the date with plus sign are the days after this date.
3 PHARMACEUTICAL FORM
Radionuclide generator
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
The eluate from the generator (sodium pertechnetate [99mTc] injection (fission), European Pharmacopoeia), may be used as a reagent for labelling of various carrier compounds supplied as kits or administered directly in vivo.
When administered intravenously, the sterile sodium pertechnetate [99mTc] solution is used as a diagnostic aid in the following: a) Thyroid scintigraphy: direct imaging and measurement of thyroid uptake to give information on the size, position, nodularity and function of the gland in thyroid disease.
b) Salivary gland scintigraphy: to assess salivary gland function and duct patency.
c) Location of ectopic gastric mucosa: Meckel's diverticulum.
d) Cerebral scintigraphy: to identify breaches in the blood-brain barrier caused by tumour, infarction, haemorrhage and oedema, when no other methods are available.
When used in conjunction with pre-treatment with a reducing agent to effect technetium-99m labelling of red blood cells:
e) Cardiac and vascular scintigraphy
- angiocardioscintigraphy for :
. evaluation of ventricular ejection fraction
. evaluation of global and regional cardiac wall motion
. myocardial phase imaging
- organ perfusion or vascular abnormalities imaging.
f) Diagnosis and localisation of occult gastrointestinal bleeding
Following instillation of sterile sodium pertechnetate [99mTc] into the eye: g) Lachrymal duct scintigraphy: to assess patency of tear ducts.
4.2 Posology and method of administration
Sodium pertechnetate [99mTc] is normally administered intravenously at activities which vary widely according to the clinical information required and the equipment employed. Pre-treatment of patients with thyroid blocking agents or reducing agents may be necessary for certain indications.
Recommended activities are as follows: - Adults and the elderly:
Thyroid scintigraphy: 18.5 - 80 MBq (0.5 - 2.2 mCi)
Scintigraphy performed 20 minutes after intravenous injection.
Salivary gland scintigraphy: 40 MBq (1.1 mCi)
Scintigraphy performed immediately after intravenous injection and at regular intervals up to 15 minutes.
Meckel's diverticulum scintigraphy: 400 MBq (10.8 mCi)
Scintigraphy performed immediately after intravenous injection and at regular intervals up to 30 minutes.
Brain scintigraphy: 370 - 800 MBq (10 - 21.6 mCi)
Rapid sequential images are taken immediately within the first minute after intravenous administration; static images 1 to 4 hours later. Thyroid and choroid plexus should be blocked to avoid non-specific technetium-99m uptake.
Cardiac and vascular scintigraphy: 740 - 925 MBq (20 - 25 mCi)
Red cells are labelled in vivo or in vitro by pre-treating with a reducing agent. Dynamic images are taken in the first minute after intravenous administration, followed by regular images over 30 minutes.
Gastrointestinal bleeding: 740 - 925 MBq (20 - 25 mCi)
Red cells are labelled in vivo or in vitro by pre-treating with a reducing agent. Dynamic images are taken in the first minute after intravenous administration, followed by regular images at appropriate intervals for up to 24 hours.
Lachrymal duct scintigraphy: 2 - 4 MBq (0.05 - 0.11 mCi) each eye
Drops are instilled into the eye and dynamic images are taken over 2 minutes, followed by static images at appropriate intervals over 20 minutes.
Children:
The activity for administration to children may be calculated from the recommended range of adult activity and adjusted according to body weight or surface area.
However, the Paediatric Task Group of EANM recommends that the activity to be administered to a child should be calculated from the body weight according to the following table:
Fraction of adult dose:
|
3 kg |
= 0.1 |
22 |
kg |
= 0.50 |
42 kg |
= 0.78 |
|
4 kg |
= 0.14 |
24 |
kg |
= 0.53 |
44 kg |
= 0.80 |
|
6 kg |
= 0.19 |
26 |
kg |
= 0.56 |
46 kg |
= 0.82 |
|
8 kg |
= 0.23 |
28 |
kg |
= 0.58 |
48 kg |
= 0.85 |
|
10 kg |
= 0.27 |
30 |
kg |
= 0.62 |
50 kg |
= 0.88 |
|
12 kg |
= 0.32 |
32 |
kg |
= 0.65 |
52-54 kg |
= 0.90 |
|
14 kg |
= 0.36 |
34 kg |
|
16 kg |
= 0.40 |
36 kg |
|
18 kg |
= 0.44 |
38 kg |
|
20 kg |
= 0.46 |
40 kg |
|
0.68 |
56-58 kg |
= 0.92 |
|
0.71 |
60-62 kg |
= 0.96 |
|
0.73 |
64-66 kg |
= 0.98 |
|
0.76 |
68 kg |
= 0.99 |
In very young children (up to 1 year) a minimum dose of 20 MBq (0.54 mCi) (10 MBq - 0.27 mCi - in thyroid scintigraphy) for direct administration or 80 MBq (2.2 mCi) for red blood cell labelling is necessary in order to obtain images of sufficient quality.
4.3 Contraindications
None known
4.4 Special warnings and precautions for use
Radiopharmaceutical agents should be used only by qualified personnel with the appropriate government authorisations for the use and manipulations of radionuclides.
This radiopharmaceutical may be received, used and administered only by authorised personnel in designated clinical settings. Its receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licences of local competent official organisations.
Radiopharmaceuticals should be prepared by the user in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken complying with the requirements of Good Pharmaceutical Manufacturing Practice for radiopharmaceuticals.
4.5 Interaction with other medicinal products and other forms of interaction
Drug interactions have been reported in brain scintigraphy where there can be increased uptake of pertechnetate [99mTc] in the walls of cerebral ventricles as a result of methotrexate-induced ventriculitis. In abdominal imaging, drugs such as atropine, isoprenaline and analgesics can result in a delay in gastric emptying and redistribution of pertechnetate.
4.6 Pregnancy and lactation
Technetium-99m (as free pertechnetate) has been shown to cross the placental barrier.
Where it is necessary to administer radioactive medicinal products to a woman of childbearing potential, information should always be sought about pregnancy. Any woman who has missed a period should be assumed to be pregnant until proven otherwise. Where uncertainty exists, it is particularly important that the radiation exposure should be the minimum consistent with achieving the desired clinical
information. Alternative techniques which do not involve ionising radiations should be considered.
Radionuclide procedures carried out on pregnant women also involve radiation doses to the foetus. Only imperative investigations should be carried out during pregnancy, when the likely benefit exceeds the risk incurred by the mother and the foetus. Direct administration of 800 MBq (21.6 mCi) sodium pertechnetate [99mTc] to a patient results in an absorbed dose to the uterus of 6.5 mGy. Following pre-treatment of patients with a blocking agent, administration of 800 MBq (21.6 mCi) sodium pertechnetate [99mTc] results in an absorbed dose to the uterus of 5.3 mGy. Administration of 925 MBq (25 mCi) 99mTc-labelled red blood cells results in an absorbed dose to the uterus of 4.3 mGy. Doses above 0.5 mGy should be regarded as a potential risk to the foetus.
Before administering a radioactive medicinal product to a woman who is breast feeding, consideration should be given as to whether the investigation could be reasonably delayed until the mother has ceased breast feeding and as to whether the most appropriate choice of radiopharmaceutical has been made. If the administration is considered necessary, breast feeding should be interrupted and the expressed feeds discarded. Breast feeding can be restarted when the activity level in the milk will not result in a radiation dose to the child greater than 1 mSv.
4.7 Effects on ability to drive and use machines
Effects on ability to drive and use machines have not been described.
4.8 Undesirable effects
The following table presents how the frequencies are reflected in this section:
Very common (>1/10)
Common (>1/100 to <1/10)
Uncommon (>1/1,000 to <1/100)
Rare (>1/10,000 to <1/1,000)
Very rare (<1/10,000)
Not known (cannot be estimated from the available data)
|
MedDRA Body system SOCs |
Symptoms |
Frequency |
|
Immune system disorders |
Hypersensitivity |
Not known |
|
Nervous system disorders |
Coma |
Not known |
|
Cardiac disorders |
Arrhythmia |
Not known |
|
Vascular disorders |
Vasodilatation |
Not known |
|
Skin and subcutaneous tissue |
Urticaria |
Not known |
|
disorders |
Pruritus |
Not known |
|
General disorders and |
Face oedema |
Not known |
|
administration site conditions |
Extravasation |
Not known |
Allergic reactions have been reported following intravenous injection of sodium pertechnetate [99mTc] and include urticaria, facial oedema, vasodilatation, pruritus, cardiac arrhythmia and coma.
Extravasation reactions have been reported.
For each patient, exposure to ionising radiations must be justifiable on the basis of likely clinical benefit. The activity administered must be such that the resulting radiation dose is as low as reasonably achievable bearing in mind the need to obtain the intended diagnosis or therapeutic result.
Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects. For diagnostic nuclear medicine investigations, the current evidence suggests that these adverse effects will occur with low frequency because of the low radiation doses incurred.
For most diagnostic investigations using a nuclear medicine procedure, the radiation dose delivered is less than 20 mSv (EDE). Higher doses may be justified in some clinical circumstances.
4.9 Overdose
In the event of the administration of a radiation overdose with sodium pertechnetate [99mTc], the absorbed dose should be reduced where possible by increasing the elimination of the radionuclide from the body. Measures to reduce possible harmful effects include frequent voiding of urine and promotion of dieresis and faecal excretion.
Very little supportive treatment can be undertaken in the event of an overdose of 99mTc-labelled red blood cells since elimination is dependent on the normal haemolytic process.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
No pharmacological activity has been observed in the range of doses administered for diagnostic purposes.
5.2 Pharmacokinetic properties
The pertechnetate ion has similar biological distribution to iodide and perchlorate ions, concentrating temporarily in salivary glands, choroid plexus, stomach (gastric mucosa) and in the thyroid gland, from which it is released unchanged. The pertechnetate ion also tends to concentrate in areas with increased vascularisation or with abnormal vascular permeability, particularly when pre-treatment with blocking agents inhibits uptake in glandular structures. Technetium-99m is selectively excluded from the cerebrospinal fluid.
Following intravenous administration, pertechnetate [99mTc] is distributed throughout the vascular system from which it is cleared by three main mechanisms :
rapid removal, depending on the diffusion equilibrium with interstitial fluid,
intermediate rate of removal, depending on the concentration of the pertechnetate in glandular tissues, mainly thyroid, salivary and gastric fundus glands which have an ionic pump mechanism, slow removal, by glomerular filtration by the kidneys, dependent on rate of urinary excretion.
Plasma clearance has a half-life of approximately 3 hours.
Excretion during the first 24 hours following administration is mainly urinary (approximately 25 %) with faecal excretion occurring over the next 48 hours. Approximately 50 % of the administered activity is excreted within the first 50 hours.
When selective uptake of pertechnetate [99mTc] in glandular structures is inhibited by the pre-administration of blocking agents, excretion follows the same pathways but there is a higher rate of renal clearance.
When pertechnetate [99mTc] is administered in association with pre-treatment with reducing agents such as stannous medronate or stannous pyrophosphate which cause a "stannous loading" of red blood cells, up to approximately 95 % of the administered activity is taken up by the red blood cells where it becomes bound within the cells. Any unbound pertechnetate [99mTc] is cleared by the kidneys; radioactivity in the plasma normally constitutes less than 5 % of the intravascular activity.
The fate of the technetium-99m follows that of the labelled erythrocytes themselves and the activity is cleared very slowly. A small level of elution of activity from the circulating red cells is thought to occur.
5.3 Preclinical safety data
a) There is no information on acute, subacute and chronic toxicity from single or repeated dose administration.
b) Reproductive toxicity
Placental transfer of technetium-99m from intravenous administered sodium pertechnetate [99mTc] has been studied in mice. The pregnant uterus was found to contain as much as 60 % of the injected technetium-99m when administered without perchlorate pre-administration. Studies performed on pregnant mice during gestation, gestation and lactation, and lactation alone showed changes in progeny which included weight reduction, hairlessness and sterility.
5.4. Radiation dosimetry
According to IRCP 53, the radiation doses absorbed by a patient following direct administration of sodium pertechnetate [99mTc] are as follows:
(i) Without pre-treatment with blocking agent:
|
Absorbed dose per unit activity administered (mGy/MBq) | |||||||||||||||||||
|
Organ |
Adult |
15 years |
10 years |
5 |
years |
1 year | |||||||||||||
|
Adrenals |
3 |
.6 |
X |
10-3 |
4 |
7 |
X |
10-3 |
7 |
.1 |
X |
10-3 |
1 |
1 |
X |
10-2 |
1.9 |
X |
10-2 |
|
Bladder wall |
1 |
.9 |
X |
10-2 |
2 |
3 |
X |
10-2 |
3 |
4 |
X |
10-2 |
5 |
1 |
X |
10-2 |
9.1 |
X |
10-2 |
|
Bone surfaces |
3 |
.9 |
X |
10-3 |
4 |
7 |
X |
10-3 |
6 |
9 |
X |
10-3 |
1 |
0 |
X |
10-2 |
1.9 |
X |
10-2 |
|
Breast |
2 |
.3 |
X |
10-3 |
2 |
3 |
X |
10-3 |
3 |
5 |
X |
10-3 |
5 |
7 |
X |
10-3 |
1.1 |
X |
10-2 |
|
Gastro-intestinal tract | |||||||||||||||||||
|
Stomach wall |
2 |
.9 |
X |
10-2 |
3 |
6 |
X |
10-2 |
5 |
0 |
X |
10-2 |
8 |
1 |
X |
10-2 |
1.5 |
X |
10-1 |
|
Small intestine |
1 |
8 |
X |
10-2 |
2 |
2 |
X |
10-2 |
3 |
4 |
X |
10-2 |
5 |
2 |
X |
10-2 |
9.0 |
X |
10-2 |
|
Upper large intestine |
6.2 |
X |
10-2 |
7.7 |
X |
10-2 |
1.3 |
X |
10-1 |
2.1 |
X |
10-1 |
3.9 |
X |
10-1 |
|
wall | |||||||||||||||
|
Lower large intestine |
2.2 |
X |
10-2 |
2.8 |
X |
10-2 |
4.6 |
X |
10-2 |
7.4 |
X |
10-2 |
1.4 |
X |
10-1 |
|
wall | |||||||||||||||
|
Kidneys |
5.0 |
X |
10-3 |
6.0 |
X |
10-3 |
8.7 |
X |
10-3 |
1.3 |
X |
10-2 |
2.1 |
X |
10-2 |
|
Liver |
3.9 |
X |
10-3 |
4.8 |
X |
10-3 |
8.0 |
X |
10-3 |
1.3 |
X |
10-2 |
2.2 |
X |
10-2 |
|
Lungs |
2.7 |
X |
10-3 |
3.4 |
X |
10-3 |
5.1 |
X |
10-3 |
7.9 |
X |
10-3 |
1.4 |
X |
10-2 |
|
Ovaries |
1.0 |
X |
10-2 |
1.3 |
X |
10-2 |
1.9 |
X |
10-2 |
2.7 |
X |
10-2 |
4.5 |
X |
10-2 |
|
Pancreas |
5.9 |
X |
10-3 |
7.2 |
X |
10-3 |
1.1 |
X |
10-2 |
1.6 |
X |
10-2 |
2.7 |
X |
10-2 |
|
Salivary glands |
9.3 |
X |
10-3 |
1.2 |
X |
10-2 |
1.7 |
X |
10-2 |
2.4 |
X |
10-2 |
3.9 |
X |
10-2 |
|
Red marrow |
6.1 |
X |
10-3 |
7.1 |
X |
10-3 |
9.8 |
X |
10-3 |
1.3 |
X |
10-2 |
2.0 |
X |
10-2 |
|
Spleen |
4.4 |
X |
10-3 |
5.3 |
X |
10-3 |
7.9 |
X |
10-3 |
1.2 |
X |
10-2 |
2.1 |
X |
10-2 |
|
Testes |
2.7 |
X |
10-3 |
3.7 |
X |
10-3 |
5.9 |
X |
10-3 |
9.3 |
X |
10-3 |
1.7 |
X |
10-2 |
|
Thyroid |
2.3 |
X |
10-2 |
3.7 |
X |
10-2 |
5.6 |
X |
10-2 |
1.2 |
X |
10-1 |
2.3 |
X |
10-1 |
|
Uterus |
8.1 |
X |
10-3 |
1.0 |
X |
10-2 |
1.6 |
X |
10-2 |
2.4 |
X |
10-2 |
4.0 |
X |
10-2 |
|
Other tissue |
3.4 |
X |
10-3 |
4.0 |
X |
10-3 |
6.0 |
X |
10-3 |
9.3 |
X |
10-3 |
1.7 |
X |
10-2 |
|
Effective Dose Equivalent (mSv/MBq) |
1.3 |
X |
10-2 |
1.6 |
X |
10-2 |
2.5 |
X |
10-2 |
4.0 |
X |
10-2 |
7.3 |
X |
10-2 |
The effective dose equivalent resulting from an administered activity of 800 MBq (21.6 mCi) sodium pertechnetate [99mTc] is 10.4 mSv.
(ii) With pre-treatment with blocking agent:
|
Absorbed dose per unit activity (mGy/MBq) when blocking agents are given | |||||
|
Organ |
Adult |
15 years |
10 years |
5 years |
1 year |
|
Adrenals |
3.3 x 10-3 |
4.1 x 10-3 |
6.3 x 10-3 |
9.5 x 10-3 |
1.7 x 10-2 |
|
Bladder wall |
3.2 x 10-2 |
3.9 x 10-2 |
5.7 x 10-2 |
8.4 x 10-2 |
1.5 x 10-1 |
|
Bone surfaces |
3.8 x 10-3 |
4.5 x 10-3 |
6.7 x 10-3 |
1.0 x 10-2 |
1.8 x 10-2 |
|
Breast |
2.5 x 10-3 |
2.5 x 10-3 |
3.6 x 10-3 |
5.7 x 10-3 |
1.1 x10-2 |
|
Gastro-intestinal tract | |||||
|
Stomach wall |
3.2 x 10-3 |
4.1 x 10-3 |
6.6 x 10-3 |
9.3 x 10-3 |
1.7 x 10-2 |
|
Small intestine |
4.1 x 10-3 |
4.9 x 10-3 |
7.6 x 10-3 |
1.1 x 10-2 |
2.0 x 10-2 |
|
Upper large intestine |
3.8 x 10-3 |
4.9 x 10-3 |
7.1 x 10-3 |
1.1 x 10-2 |
1.9 |