Emerade 150 Micrograms Solution For Injection In Pre-Filled Pen
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Emerade, 150 micrograms, solution for injection in pre-filled pen
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The pre-filled pen contains 0.5 ml of adrenaline solution 1 mg/ml.
Emerade 150 micrograms delivers a single dose of 0.15 ml containing 150 micrograms of adrenaline (as tartrate).
Each 0.15 ml (150 micrograms) dose contains 0.075 mg sodium meta-bisulphite (E223). For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection in a pre-filled pen (auto-injector). Clear and colourless solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Emerade is indicated for the emergency treatment of severe acute allergic reactions (anaphylaxis) triggered by allergens in foods, medicines, insect stings or bites, and other allergens as well as for exercise-induced or idiopathic anaphylaxis.
4.2 Posology and method of administration
Posology
The effective dose is usually within the range 5-10 micrograms per kg bodyweight but higher doses may be necessary in some cases.
Paediatric population
Use in children: Emerade 500 micrograms is not recommended for use in children.
Children below 15 kg bodyweight
A dosage below 150 micrograms cannot be administered with sufficient accuracy in children weighing less than 15 kg and use is therefore not recommended unless during a life-threatening situation and under medical advice.
Children between 15 kg and 30 kg bodyweight The usual dose is 150 micrograms.
Children over 30 kg bodyweight The usual dose is 300 micrograms.
Adolescent patients over 30 kg bodyweight
The dosage recommendations for adult patients should be followed.
Use in adults
The recommended dose is 300 micrograms for individuals under 60 kg in body weight. The recommended dose is 300 to 500 micrograms for individuals over 60 kg body weight depending on clinical judgement.
An initial dose should be administered as soon as symptoms of anaphylaxis are recognised. In the absence of clinical improvement or if deterioration occurs, a second injection with an additional Emerade may be administered 5 - 15 minutes after the first injection. It is recommended that the patients are prescribed two Emerade pens which they should carry at all times.
Method of administration
For intramuscular injection only.
For single use.
Emerade is given intramuscularly as soon as the symptoms of anaphylactic shock arise. A poor outcome from anaphylaxis is associated with late administration of adrenaline.
Emerade must be injected in the outer side of the thigh.
Massaging around the injection area accelerates absorption.
The injection can be administered through clothing.
The patient/carer should be informed that following each use of Emerade:
■ They should call for immediate medical assistance, ask for an ambulance and state ‘anaphylaxis’ even if symptoms appear to be improving (see section 4.4).
■ Conscious patients should preferably lie flat with feet elevated but sit up if they have breathing difficulties. Unconscious patients should be placed on their side in the recovery position.
■ The patient should if possible remain with another person until medical assistance arrives.
For detailed instruction for use, refer to section 6.6.
4.3 Contraindications
There are no absolute contraindications to the use of Emerade in an allergic emergency.
4.4 Special warnings and precautions for use
Do not remove the needle shield until ready for use.
Emerade must be administrated only into the anterolateral thigh.
The injection is delivered immediately after the triggering cylinder is pressed against the skin. Patients should be advised not to inject Emerade into the gluteus maximus due to the risk of accidental injection into a vein.
Emerade should be used in emergency situations as life-sustaining treatment.
The patient must urgently seek medical assistance for further treatment after using Emerade.
All patients who are prescribed Emerade should be thoroughly instructed to understand the indications for the use and the correct method of administration (see section 6.6). It is strongly advised also to educate the patient’s immediate associates (e.g. parents, caregivers, teachers) for the correct usage of Emerade in case support is needed in the emergency situation.
The patient/carer should be informed about the possibility of biphasic anaphylaxis which is characterised by initial resolution followed by recurrence of symptoms some hours later. Patients with concomitant asthma may be at increased risk of a severe anaphylactic reaction.
Use with caution in patients with heart diseases including angina pectoris, cardiac arrhythmia, cor pulmonale, obstructive cardiomyopathy and atherosclerosis. There is also a risk for adverse reactions after the administration of adrenaline to patients with hyperthyroidism, hypertension, phaeochromocytoma, glaucoma, severe renal impairment, prostate adenoma, hypercalcaemia, hypokalaemia, diabetes, and in elderly patients and pregnant women.
Emerade contains sodium metabisulphite which can cause allergic reactions including anaphylaxis and bronchospasm in sensitive individuals particularly in those with a history of asthma. All those patients should be carefully instructed in which circumstances Emerade must be used.
Unintentional injection in hands and feet can result in peripheral ischemia that may require treatment.
Patients should be warned regarding related allergens and should be investigated whenever possible so that their specific allergens can be characterised.
Emerade is essentially sodium free (contains less than 1 mmol sodium (23 mg) per dose).
4.5 Interaction with other medicinal products and other forms of interaction
Certain medicines can enhance the effect of adrenaline: Tricyclic antidepressants, monoamine oxidase (MAO) inhibitors, and catechol-O-methyl transferase (COMT) inhibitors. Adrenaline must be used with caution in patients receiving halogenated
hydrocarbons and related medicines and drugs that may sensitize the heart to arrhythmias, e.g. digitalis, quinidine, halogenated anaesthetics.
The administration of fast-acting vasodilators or a-blockers can counteract the effects of adrenaline on blood pressure. P-blockers can inhibit the stimulating effect of adrenaline.
The hyperglycaemic effect of adrenaline may necessitate an increase in insulin or oral hypoglycaemic treatment in diabetic patients.
4.6 Fertility, Pregnancy and lactation
There are no adequate or well-controlled studies of adrenaline during pregnancy. Adrenaline should be used in pregnancy only when the potential benefit to the mother outweighs the possible risk to the foetus.
Because of its poor oral bioavailability and short half-life, any adrenaline in breast milk is unlikely to affect the nursing infant.
4.7 Effects on ability to drive and use machines
Emerade has no or negligible influence on the ability to drive and use machines, however, patients are not recommended to drive or use machines following administration of adrenaline, since they will be affected by the anaphylactic reaction.
4.8 Undesirable effects
Side-effects of adrenaline in general are associated with the a- and P-receptor activity of adrenaline.
The following table is based upon experience with the use of adrenaline.
The adverse events were classified according to the following frequencies:
Very common (>1/10)
Common (>1/100, <1/10)
Uncommon (>1/1,000, <1/100)
Rare (>1/10,000 to <1/1,000)
Very rare (<1/10,000)
Not known (cannot be estimated from the available data).
Emerade contains sodium metabisulphite, which may rarely cause severe hypersensitivity reactions (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:
United Kingdom
Yellow Card Scheme; Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
An overdose, or an accidental intravascular injection of adrenaline, can originate a sudden increase in blood pressure that can cause cerebral haemorrhage. Severe pulmonary oedema caused by peripheral vasoconstriction together with cardiac stimulation can result in death. Severe pulmonary oedema with difficulty in breathing can be treated with fast-acting a-blockers. Life-threatening heart arrhythmias can be treated with p-blocking agents.
5 PHARMACOLOGICAL PROPERTIES
5.1
|
System organ class |
Frequency |
Adverse reaction |
|
Metabolic and nutrition disorders |
Not known |
Hyperglycaemia, hypokalaemia, acidosis |
|
Psychiatric disorders |
Not known |
Anxiety, hallucination |
|
Nervous system disorders |
Not known |
Headache, dizziness, tremor, syncope |
|
Cardiac disorders |
Not known |
Tachycardia, arrhythmia, palpitations, angina pectoris, stress cardiomyopathy |
|
Vascular disorders |
Not known |
Hypertension, vasoconstriction, peripheral ischaemia |
|
Respiratory, thoracic and mediastinal disorders |
Not known |
Bronchospasm |
|
Gastrointestinal disorders |
Not known |
Nausea, vomiting |
|
General disorders and administration site conditions |
Not known |
Hyperhidrosis, asthenia |
Pharmacodynamic properties
Pharmacotherapeutic group: Cardiac stimulants excl. cardiac glycosides - Adrenergic and dopaminergic agents - Adrenaline, ATC-code: C01CA24
Adrenaline is the natural active sympathomimetic hormone from the adrenal medulla. It stimulates both the a- and P-adrenergic receptors. Adrenaline is the first choice for emergency treatment of severe allergic reactions and idiopathic or exercised-induced anaphylaxis.
Adrenaline has a potent vasoconstrictive effect through its a-adrenergic stimulation. This effect counteracts the vasodilatation and increased vascular perfusion, leading to low intravascular flow and hypotension, which are the main pharmacotoxicological effects in the anaphylactic shock.
By stimulating P-receptors in the lungs, adrenaline produces a potent bronchodilator effect with relief of wheezing and dyspnea. Adrenaline also relieves pruritus, urticaria and angioedema associated to anaphylaxis.
5.2 Pharmacokinetic properties
Circulating adrenaline is metabolized in the liver and other tissues by the enzymes COMT and MAO. Inactive metabolites are excreted in the urine.
The half-life of adrenaline in plasma is about 2 to 3 minutes. However, when adrenaline is injected subcutaneously or intramuscularly the absorption is retarded by local vasoconstriction and thus the effects can last longer than as predicted by halflife. Massage around the injection site is advised to accelerate absorption.
5.3 Preclinical safety data
Adrenaline has been extensively used in the emergency treatment of severe allergic reactions for many years. There is no further preclinical data relevant for prescribers besides those already described in this SmPC.
6 PHARMACEUTICAL PARTICULARS
6.1
List of excipients
Sodium chloride
Sodium meta-bisulfite (E223)
Disodium edetate
Hydrochloric acid (for adjustment of pH) Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
30 months
6.4 Special precautions for storage
Store in the original package, a specially designed case to protect the pen and the labelling.
Store below 25°C. Do not freeze.
6.5 Nature and contents of container
Emerade consists of a pre-filled syringe made of glass with a polyisoprene rubber needle plunger in an auto-injector. Emerade is latex free.
Exposed needle length:
Emerade 150 micrograms: 16 mm
Emerade 300 micrograms and Emerade 500 micrograms: 23 mm Package
Emerade has a plastic outer carton to store the auto-injector in.
Pack sizes: 1 or 2 pre-filled pens.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
It is very important that the patient receives detailed information on how to use Emerade.
For single use only.
The expiry date is indicated on the label and Emerade should not be used after this date. Discard and replace the auto-injector after expiry date.
Check the solution periodically through the inspection window of the unit by lifting the label to make sure the solution is clear and colourless. Discard and replace Emerade if the solution is discoloured or contains particles.
Emerade should always be carried if at risk of anaphylaxis.
Emerade is designed for easy use and has to be considered as a first aid. Emerade should be used for intramuscular injection only on the outer thigh. The injection occurs when the triggering cylinder is gently pressed into the thigh. This can be done through clothing. Emerade has an opening only at the needle end and none at the opposite end.
Method of administration
The instructions for use must be carefully followed in order to avoid accidental injection.
It is recommended that your family members, carers or teachers are also instructed in the correct use of Emerade.
Emerade should only be used for injection in the outer thigh. The injection occurs when Emerade is pressed into the thigh. This can be done through clothing.
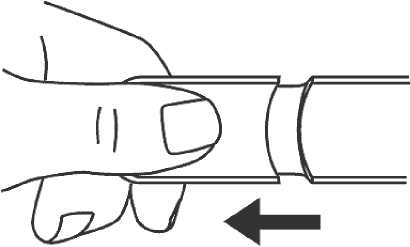
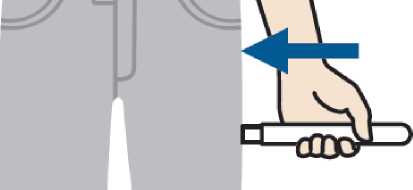
Press
1. Remove the needle shield.
2. Place and press Emerade against the outer side of the thigh. A click can be heard when the injection goes into the muscle.
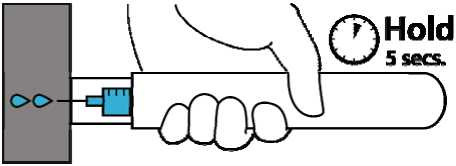
3. Hold Emerade against the thigh for about 5 seconds. Lightly massage the injection site afterwards.
Seek immediate medical help.
The needle in Emerade is protected before, during and after the injection.
When the injection is completed the plunger is visible in the inspection window by lifting the label.
Sometimes a single dose of adrenaline may not be sufficient to completely reverse the effects of a serious allergic reaction. For this reason, your doctor is likely to prescribe more than one Emerade for you. If your symptoms have not improved or have deteriorated within 5-15 minutes after the first injection, either you or the person with you should give a second injection. For this reason you should carry more than one Emerade with you at all times.
Emerade is intended only for emergency treatment. You must always contact your doctor or go to the nearest hospital for further treatment. Inform your doctor that you have taken an injection of adrenaline. Take the used auto-injector with you.
See Section 4.2 for instructions to be conveyed to the patient/carer regarding actions to be taken following each use of Emerade.
Do not remove the needle shield unless injection is required.
Some liquid remains in the auto-injector after the injection. The auto-injector cannot be reused.
Discard Emerade in accordance with local requirements.
Instructions for use are shown on the label, package and package leaflet.
Autoinjectors without needles are available for training purposes.
7. MARKETING AUTHORISATION HOLDER
PharmaSwiss Ceska republika s.r.o.
Jankovcova 1569/2c 170 00 Prague 7 Czech Republic
8. MARKETING AUTHORISATION NUMBER
PL 33616/0013
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
Date of first authorisation: 03 January 2013
10 DATE OF REVISION OF THE TEXT
19/09/2016