Escitalopram 5 Mg Film-Coated Tablets
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Escitalopram 5 mg film-coated tablets Escitalopram 10 mg film-coated tablets Escitalopram 20 mg film-coated tablets
Escitalopram oxalate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Escitalopram Tablets is and what it is used for
2. What you need to know before you take Escitalopram Tablets
3. How to take Escitalopram Tablets
4. Possible side effects
5. How to store Escitalopram Tablets
6. Contents of the pack and other information
1. WHAT ESCITALOPRAM TABLETS IS AND WHAT IT IS USED FOR
Escitalopram Tablets contains the active substance escitalopram. Escitalopram Tablets belongs to a group of antidepressants called selective serotonin reuptake inhibitors (SSRIs). These medicines act on the serotonin-system in the brain by increasing the serotonin level. Disturbances in the serotonin- system are considered an important factor in the development of depression and related diseases.
Escitalopram Tablets contains escitalopram and is used to treat depression (major depressive episodes) and anxiety disorders (such as panic disorder with or without agoraphobia, social anxiety disorder, generalised anxiety disorder and obsessive-compulsive disorder).
It may take a couple of weeks before you start to feel better. Continue to take Escitalopram Tablets, even if it takes some time before you feel any improvement in your condition.
You must talk to a doctor if you do not feel better or if you feel worse.
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE ESCITALOPRAM TABLETS Do not take Escitalopram Tablets
• If you are allergic to escitalopram or any of the other ingredients of this medicine (listed in section 6).
• If you take other medicines which belongs to a group called MAO inhibitors, including selegiline (used in the treatment of Parkinson's disease), moclobemide (used in the treatment of depression) and linezolid (an antibiotic).
• If you are born with or have had an episode of abnormal heart rhythm (seen at ECG; an examination to evaluate how the heart is functioning)
• If you take medicines for heart rhythm problems or that may affect the heart's rhythm (see section 2 “Other medicines and Escitalopram Tablets")
Warnings and precautions
Talk to your doctor or pharmacist before taking Escitalopram Tablets. Please tell your doctor if you have any other condition or illness, as your doctor may need to take this into consideration. In particular, tell your doctor:
• If you have epilepsy. Treatment with Escitalopram Tablets should be stopped if seizures occur for the first time, or if there is an increase in the seizure frequency (see also section 4 “Possible side effects").
• If you suffer from impaired liver or kidney function. Your doctor may need to adjust your dosage.
• If you have diabetes. Treatment with Escitalopram Tablets may alter glycaemic control. Insulin and/or oral hypoglycaemic dosage may need to be adjusted.
• If you have a decreased level of sodium in the blood.
• If you have a tendency to easily develop bleedings or bruises.
• If you are receiving electroconvulsive treatment.
• If you have coronary heart disease.
• If you suffer or have suffered from heart problems or have recently had a heart attack
• If you have a low resting heart-rate and/or you know that you may have salt depletion as a result of
prolonged severe diarrhoea and vomiting (being sick) or usage of diuretics (water tablets)
• If you experience a fast or irregular heartbeat, fainting, collapse or dizziness on standing up, which may indicate abnormal functioning of the heart rate
• If you have or have previously had eye problems, such as certain kinds of glaucoma (increased pressure in the eye).
Please note
Some patients with manic-depressive illness may enter into a manic phase. This is characterized by unusual and rapidly changing ideas, inappropriate happiness and excessive physical activity. If you experience this, contact your doctor.
Symptoms such as restlessness or difficulty to sit or stand still can also occur during the first weeks of the treatment. Tell your doctor immediately if you experience these symptoms.
Thoughts of suicide and worsening of your depression or anxiety disorder
If you are depressed and/or have anxiety disorders you can sometimes have thoughts of harming or killing yourselves. These may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
• If you have previously had thoughts about killing or harming yourself.
• If you are a young adult. Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straight away.
You may find it helpful to tell a relative or close Mend that you are depressed or have an anxiety disorder, and ask them to read this leaflet. You might ask them to tell you if they think your depression or anxiety is getting worse, or if they are worried about changes in your behaviour.
• “Reversible, selective MAO-A inhibitors”, containing moclobemide (used to treat depression).
• “Irreversible MAO-B inhibitors”, containing selegiline (used to treat Parkinson's disease). These increase the risk of side effects.
• The antibiotic linezolid.
• Lithium (used in the treatment of manic-depressive disorder) and tryptophan.
• Imipramine and desipramine (both used to treat depression).
• Sumatriptan and similar medicines (used to treat migraine) and tramadol (used against severe pain). These increase the risk of side effects.
• Cimetidine, lansoprazole and omeprazole (used to treat stomach ulcers), fluvoxamine (antidepressant) and ticlopidine (used to reduce the risk of stroke). These may cause increased blood levels of Escitalopram.
• St. John's Wort (hypericum perforatum) - a herbal remedy used for depression.
• Acetylsalicylic acid (aspirin) and non-steroidal anti-inflammatory drugs (medicines used for pain relief or to thin the blood, so called anti-coagulant). These may increase bleeding- tendency.
• Warfarin, dipyridamole, and phenprocoumon (medicines used to thin the blood, so called anti-coagulant). Your doctor will probably check the coagulation time of your blood when starting and
discontinuing Escitalopram Tablets in order to verify that your dose of anti-coagulant is still adequate.
• Mefloquin (used to treat Malaria), bupropion (used to treat depression) and tramadol (used to treat severe pain) due to a possible risk of a lowered threshold for seizures.
• Neuroleptics (medicines to treat schizophrenia, psychosis) and antidepressants (tricyclic antidepressants and SSRIs)due to a possible risk of a lowered threshold for seizures.
• Flecainide, propafenone, and metoprolol (used in cardio-vascular diseases) clomipramine, and nortriptyline (antidepressants) and risperidone, thioridazine, and haloperidol (antipsychotics).
The dosage of Escitalopram may need to be adjusted.
• Medicines that decrease blood levels of potassium or magnesium as these conditions increase the risk of life-threatening heart rhythm disorder.
Do not take Escitalopram if you take medicines for heart rhythm problems or medicines that may affect the heart's rhythm, such as Class IA and III antiarrhythmics, antipsychotics (e.g. phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants , certain antimicrobial agents (e.g.sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, anti-malarian treatment particularly halofantrine), certain antihistamines (astemizole, mizolastine). If you have any further questions about this you should speak to your doctor.
Escitalopram Tablets with food, drink and alcohol
Escitalopram Tablets can be taken with or without food (see section 3 “How to take Escitalopram”).
As with many medicines, combining Escitalopram Tablets with alcohol is not advisable, although Escitalopram is not expected to interact with alcohol.
Pregnancy, breast-feeding and fertility
Inform your doctor if you are pregnant or planning to become pregnant. Do not take Escitalopram Tablets if you are pregnant or breast-feeding, unless you and your doctor have discussed the risks and benefits involved.
If you take Escitalopram Tablets during the last 3 months of your pregnancy you should be aware that the following effects may be seen in your newborn baby: trouble with breathing, blueish skin, fits, body temperature changes, feeding difficulties, vomiting, low blood sugar, stiff or floppy muscles, vivid reflexes, tremor, jitteriness, irritability, lethargy, constant crying, sleepiness and sleeping difficulties. If your newborn baby has any of these symptoms, please contact your doctor immediately.
Make sure your midwife and/or doctor know you are on Escitalopram Tablets. When taken during pregnancy, particularly in the last 3 months of pregnancy, medicines like Escitalopram Tablets may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately.
If used during pregnancy Escitalopram Tablets should never be stopped abruptly.
It is expected that Escitalopram Tablets will be excreted into breast milk.
Citalopram, a medicine like escitalopram, has been shown to reduce the quality of sperm in animal studies. Theoretically, this could affect fertility, but impact on human fertility has not been observed as yet.
Driving and using machines
You are advised not to drive a car or operate machinery until you know how Escitalopram Tablets affects you.
This medicinal product contains lactose.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. HOW TO TAKE ESCITALOPRAM TABLETS
Always take Escitalopram Tablets exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adults
Depression
The normally recommended dose of Escitalopram Tablets is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Panic disorder
The starting dose of Escitalopram Tablets is 5 mg as one daily dose for the first week before increasing the dose to 10 mg per day. The dose may be further increased by your doctor to a maximum of 20 mg per day.
Social anxiety disorder
The normally recommended dose of Escitalopram Tablets is 10 mg taken as one daily dose. Your doctor can either decrease your dose to 5 mg per day or increase the dose to a maximum of 20 mg per day, depending on how you respond to the medicine.
Children and adolescents under 18 years of aae
Escitalopram Tablets should normally not be used for children and adolescents under 18 years. Also, you should know that patients under 18 have an increased risk of side effects such as suicide attempts, suicidal thoughts and hostility (predominately aggression, oppositional behaviour and anger) when they take this class of medicines. Despite this, your doctor may prescribe Escitalopram Tablets for patients under 18 because he/she decides that this is in their best interest. If your doctor has prescribed Escitalopram Tablets for a patient under 18 and you want to discuss this, please go back to your doctor. You should inform your doctor if any symptoms listed above develop or worsen when patients under 18 are taking Escitalopram Tablets. Also, the long term safety effects concerning growth, maturation and cognitive and behavioural development of Escitalopram Tablets in this age group have not yet been demonstrated.
Other medicines and Escitalopram Tablets
Tell your doctor if you are taking or have recently taken any other medicines, including medicines obtained without prescription.
Tell your doctor if you are taking any of the following medicines:
• "Non-selective monoamine oxidase inhibitors (MAOIs)”, containing phenelzine, iproniazid,
isocarboxazid, nialamide, and tranylcypromine as active ingredients. If you have taken any of these medicines you will need to wait 14 days before you start taking Escitalopram Tablets. After stopping Escitalopram Tablets you must allow 7 days before taking any of these medicines.
Generalised anxiety disorder
The normally recommended dose of Escitalopram Tablets is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Obsessive-compulsive disorder
The normally recommended dose of Escitalopram Tablets is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Elderly patients (above 65 years of age)
The recommended starting dose of Escitalopram Tablets is 5 mg taken as one daily dose. The dose may be increased by your doctor to 10 mg per day.
Children and adolescents fbelow 18 years of aael
Escitalopram Tablets should not normally be given to children and adolescents. For further information please see section 2 “What you need to know before you take Escitalopram Tablets”.
You can take Escitalopram Tablets with or without food. Swallow the tablet with some water. Do not chew them, as the taste is bitter.
*
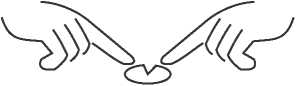
If necessary, you can divide the tablets by firstly placing the tablet on a flat surface with the score facing upwards. The tablets may then be broken by pressing down on each end of the tablet, using both forefingers as shown in the drawing.
Duration of treatment
It may take a couple of weeks before you start to feel better. Continue to take Escitalopram Tablets even if it takes some time before you feel any improvement in your condition.
Do not change the dose of your medicine without talking to your doctor first.
Continue to take Escitalopram Tablets for as long as your doctor recommends. If you stop your treatment too soon, your symptoms may return. It is recommended that treatment should be continued for at least 6 months after you feel well again.
If you take more Escitalopram Tablets than you should
If you take more than the prescribed dose of Escitalopram Tablets, contact your doctor or nearest hospital emergency department immediately. Do this even if there are no signs of discomfort. Some of the signs of an overdose could be dizziness, tremor, agitation, convulsion, coma, nausea, vomiting, change in heart rhythm, decreased blood pressure and change in body fluid/salt balance. Take the Escitalopram Tablets box/container with you when you go to the doctor or hospital.
If you forget to take Escitalopram Tablets
Do not take a double dose to make up for forgotten doses. If you do forget to take a dose, and you remember before you go to bed, take it straight away. Carry on as usual the next day. If you only remember during the night, or the next day, leave out the missed dose and carry on as usual.
If you stop taking Escitalopram Tablets
Do not stop taking Escitalopram Tablets until your doctor tells you to do so. When you have completed your course of treatment, it is generally advised that the dose of Escitalopram Tablets is gradually reduced over a number of weeks.
When you stop taking Escitalopram Tablets, especially if it is abruptly, you may feel discontinuation symptoms. These are common when treatment with Escitalopram Tablets is stopped. The risk is higher, when Escitalopram Tablets has been used for a long time or in high doses or when the dose is reduced too quickly. Most people find that the symptoms are mild and go away on their own within two weeks. However, in some patients they may be severe in intensity or they may be prolonged (2-3 months or more). If you get severe discontinuation symptoms when you stop taking Escitalopram Tablets, please contact your doctor. He or she may ask you to start taking your tablets again and come off them more slowly.
Discontinuation symptoms include: Feeling dizzy (unsteady or off-balance), feelings like pins and needles, burning sensations and (less commonly) electric shock sensations, including in the head, sleep disturbances (vivid dreams, nightmares, inability to sleep), feeling anxious, headaches, feeling sick (nausea), sweating (including night sweats), feeling restless or agitated, tremor (shakiness), feeling confused or disorientated, feeling emotional or irritable, diarrhoea (loose stools), visual disturbances, fluttering or pounding heartbeat (palpitations).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Escitalopram Tablets can cause side effects, although not everybody gets them.
The side effects usually disappear after a few weeks of treatment. Please be aware that many of the effects may also be symptoms of your illness and therefore will improve when you start to get better.
If you experience any of the following symptoms you should contact your doctor or go to the hospital straight away:
Uncommon (may affects up to 1 in 100 people):
• Unusual bleeds, including gastrointestinal bleeds Rare (may affects up to 1 in 1000 people):
• Swelling of skin, tongue, lips, or face, or have difficulties breathing or swallowing (allergic reaction),.
High fever, agitation, confusion, trembling and abrupt contractions of muscles these may be signs of a rare condition called serotonin syndrome.
Not known (frequency cannot be estimated from the available data):
• Difficulties urinating
• Seizures (fits), see also section 2 “Warnings and precautions”
• Yellowing of the skin and the white in the eyes are signs of liver function impairment/hepatitis
• Fast, irregular heart beat, fainting which could be symptoms of a life-threatening condition known as Torsade de Pointes
• Thoughts of harming or killing yourself, see also section 2 "Warnings and precautions”
In addition to above the following side effects have been reported:
Very common (may affects more than 1 in 10 people):
• Feeling sick (nausea)
• Headache
Common (may affects up to 1 in 10 people):
• Blocked or runny nose (sinusitis)
• Decreased or increased appetite
• Anxiety, restlessness, abnormal dreams, difficulties falling asleep, feeling sleepy, dizziness, yawning, tremors, prickling of the skin
• Diarrhoea, constipation, vomiting, dry mouth
• Increased sweating
• Pain in muscle and joints (arthralgia and myalgia)
• Sexual disturbances (delayed ejaculation, problems with erection, decreased sexual drive and women may experience difficulties achieving orgasm)
• Fatigue, fever
• Increased weight
Uncommon (may affects up to 1 in 100 people ):
• Nettle rash (urticaria), rash, itching (pruritus)
• Grinding one's teeth, agitation, nervousness, panic attack, confusion state
• Disturbed sleep, taste disturbance, fainting (syncope)
• Enlarged pupils (mydriasis), visual disturbance, ringing in the ears (tinnitus)
• Loss of hair
• Excessive menstrual bleeding
• Irregular menstrual period
• Decreased weight
• Fast heart beat
• Swelling of the arms or legs
• Nosebleeds
Rare (may affects up to 1 in 1000 people ):
• Aggression, depersonalisation, hallucination
• Slow heart beat
Not known (frequency can not be estimated from the available data):
• Decreased levels of sodium in the blood (the symptoms are feeling sick and unwell with weak muscles or confused)
• Dizziness when you stand up due to low blood pressure (orthostatic hypotension)
• Abnormal liver function test (increased amounts of liver enzymes in the blood)
• Movement disorders (involuntary movements of the muscles)
• Painful erections (priapism)
• Signs of increased bleeding e.g. from skin and mucous membrane (ecchymosis)
• Sudden swelling of skin or mucosa (angioedemas)
• Increase in the amount of urine excreted (inappropriate ADH secretion)
• Flow of milk in men and in women that are not nursing
• Mania
• An increased risk of bone fractures has been observed in patients taking this type of medicines.
• Alteration of the heart rhrythm (called “pronlongation of QT interval”, seen on ECG, electrical activity of the heart).
In addition, a number of side effects are known to occur with drugs that work in a similar way to escitalopram (the active ingredient of Escitalopram Tablets). These are:
• Motor restlessness (akathisia)
• Loss of appetite
Reporting of side effects:
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via www.mhra.aov.uk/vellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ESCITALOPRAM TABLETS
Keep all medicines out of the sight and reach of children.
Do not use Escitalopram Tablets after the expiry date, which is stated on the label or carton after EXP. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
If the medicine becomes discoloured or show any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Escitalopram Tablets contains
The active substance is escitalopram. Each Escitalopram tablet contains 5 mg, 10 mg or 20 mg escitalopram (as oxalate).
The other ingredients are:
Core: Lactose monohydrate, silica colloidal anhydrous, croscarmellose sodium, sodium carboxy methyl cellulose, propyl gallate, talc and magnesium stearate.
Coating: HPMC 2910/Hypromellose 6cP, titanium dioxide (E 171) and macrogol 400
What Escitalopram Tablets looks like and contents of the pack
Escitalopram Tablets is presented as 5 mg, 10 mg and 20 mg film-coated tablets. The tablets are described below.
5 mg: White to off white, circular, biconvex, film coated tablet having 'ML 59' debossed on one side, and
plain on other (approximate size: 5.5 mm).
10 mg: White to off white, circular, biconvex, film coated tablet having 'ML 60' debossed on one side, break line on opposite side and having notches on either ends of break line (approximate size: 7.14 mm).
20 mg: White to off white, circular, biconvex, film coated tablet having 'ML 61' debossed on one side, break line on opposite side and having notches on either ends of break line (approximate size: 9.5 mm).
The Escitalopram Tablets are available in Cold form laminate 25 p OPA/ 45p Alu foil /60 p PVC - Aluminium foil blister pack:
Pack sizes: 28, 56 and 98 tablets Not all pack sizes may be marketed.
Marketing Authorisation Holder and manufacturer
Wilcare Pharma Limited,
Building 6 Unit 14, Croxley Green Business Park, Watford, England, WD18 8YH
If you would like this leaflet in a different format or register a complaint please contact the Marketing Authorisation Holder listed above or send an email to complaints@wilcarepharma.co.uk
This leaflet was last revised in March 2015