Fencino 25 Micrograms/Hour Transdermal Patch
Read all of this leaflet carefully before you start using this medicine
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
INFORMATION FOR THE USER
|
® micrograms/hour transdermal patch | |
|
Fentanyl |
MOB ASHBOURNE LtdJ |
For adults and children aged 2 years and older
What is in this leaflet:
1. What Fencino® is and what it is used for
2. What you need to know before you use Fencino®
3. How to use Fencino®
4. Possible side effects
5. How to store Fencino®
6. Contents of the pack and other information
1. WHAT FENCINO® IS AND WHAT IT IS USED FOR
Fencino® is a strong painkiller; its painkilling activity is mediated through the central nervous system. Adults:
Fencino® is used for long-term management of severe and long-lasting pain that can only be managed adequately with strong pain relievers (opioid analgesics).
Children:
Fencino® is used for long-term management of severe chronic pain that can only be managed adequately with strong pain relievers (opioid analgesics) in children aged 2 years or older who have previously been treated with opioid analgesics.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE FENCINO®
Do not use Fencino®:
- if you are hypersensitive (allergic) to fentanyl, peanut, soya or any of the other ingredients of this medicine (listed in section 6).
- if you suffer from pain which lasts only for a short period, e.g. after a surgical procedure.
- if your central nervous system is severely impaired, for instance by brain injury.
- if your breathing function is severely impaired.
Warnings and precautions
Talk to your doctor before using Fencino®.
Warning
Fencino® is a medicinal product that could be life-threatening to children. This is also the case with used transdermal patches. Bear in mind that the design of this medicinal product could be tempting to a child, which in some cases may lead to a fatal outcome. Fencino® can have life-threatening side effects in persons that are not using prescribed opioid medicines on a regular basis.
This medicine should only be used under the supervision of a doctor who is experienced in the treatment of pain. Your doctor will use the treatment with Fencino® as a part of an integrated management of pain and will assess you for your individual response to Fencino® at regular intervals.
As strong pain relievers may cause breathing problems, Fencino® should be used with caution, under special supervision and at low doses in patients with:
- existing breathing problems (respiratory depression), asthma. Breathing difficulties may continue or re-occur following removal of the transdermal patch; therefore, you must be monitored for these signs. The probability of these side effects increases with increasing doses and may be increased by medicines affecting brain function (see section “Using other medicines”)
- diseases of the lungs, e.g. chronic pulmonary disease (CPD), as breathing may be reduced
- impaired liver and/or kidney function as excretion of fentanyl may be delayed.
Particular care is necessary
- if you have severe side effects. Following removal of Fencino® you should be monitored for at least 24 hours or longer, depending on your symptoms.
- if you have had a head injury, a brain tumour, signs of increased intracranial pressure, changes in your state of consciousness or loss of consciousness or coma. Strong pain relievers (opioids) may mask the course of head injury.
- if you have a too slow, irregular heartbeat (bradyarrhythmia).
- if you have low blood pressure or lack of fluid (hypovolaemia). This should be treated prior to initiation of treatment with Fencino®.
- if you are at an advanced age.
- if you suffer from long-lasting constipation.
- if you have existing or suspected loss of muscle function of the bowel: treatment with Fencino® must be stopped.
- if you suffer from myasthenia gravis (a disease causing tiredness and weakness of the muscles).
- if you are addicted to medicines or alcohol or have a history of drug abuse.
Repeated use of strong pain relievers (opioids) may lead to physical or psychological dependence. However, this is rarely seen when opioids are prescribed by a doctor (see section “Possible side effects”).
Do not cut Fencino® patches. The patches should be controlled before use. A divided, cut or in any way damaged patch should not be used.
Patch sticking to another person
Fencino® patches should only be used on the skin of the person for whom it was ordered by the doctor. Cases have been reported where a patch was accidentally stuck to a family member while in close physical contact or sharing the same bed as the patch wearer. A patch sticking to another person (particularly a child) may result in an overdose. If the patch sticks to the skin of another person, take the patch off immediately and seek medical attention.
Fever/external heat application
Information is available that the blood concentration of fentanyl may possibly increase by one third, when the skin temperature rises to 40°C. Inform your doctor if you develop a fever during the treatment.
For this reason you should avoid exposing the patch on the skin to direct heat such as heating pads, electric blankets, heated water beds, heat or tanning lamps, intensive sun bathing, hot water bottles, saunas, prolonged hot baths, or hot whirlpool spa baths.
Children and adolescents
Fencino® should not be administered to children who have not been treated with opioids before. Patients may experience a significant or life-threatening respiratory depression.
Fentanyl transdermal patch has not been studied in children under 2 years of age. Fencino® should be administered only to opioid-tolerant children age 2 years or older (please see “How to use Fencino®”). Fencino® should not be used in children under 2 years of age.
To minimise the potential of young children removing and consuming the patch, the site of administration should be chosen carefully. The adhesion of the patch should be monitored carefully and if necessary the patch should be reapplied. The patch should be applied, removed and disposed of by a clinical person, a doctor or by an adult attending the child and not by the child. Fencino® should be stored out of the reach and sight of children before and after administration.
Misuse for doping (cheating at competitive events)
The use of Fencino® can lead to positive results in doping tests. Using Fencino® for doping reasons can result in a risk to your health.
Other medicines and Fencino®
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This includes medicines that you buy without a prescription.
If you take any of the following medicines, an interactive increase of the central depression is to be expected and side effects like difficulty in breathing, low blood pressure, severe sleepiness/ coma and death are possible:
- strong painkillers
- depressants and hypnotics
- medicines used to put you to sleep or for relaxation of your muscles (in the case of a planned medical or dental procedure with anaesthesia/narcosis, please inform your doctor or dentist that you are using Fencino®
- phenothiazines (medicines to treat neuroleptic disease)
- medicines used for treating anxiety
- medicines used for treating allergies (antihistamines that make you feel tired)
- alcohol
The concomitant use with specific medicines (CYP3A4 inhibitors) may reduce the breakdown of fentanyl in the liver which could increase or prolong the therapeutic effects and side effects, e.g.:
- ritonavir, nefinavir (medicines to treat viruses)
- ketoconazole, itraconazole, fluconazole, voriconazole (medicines to treat fungal diseases)
- troleandomycin, clarithromycin (antibiotics)
- nefazodone (medicine to treat depression)
- verapamil, diltiazem (medicines to treat heart diseases and high blood pressure)
- amiodarone (medicine to treat a heart problem called arrhythmia)
Concomitant use of specific medicines (CYP3A4 inducers) may increase breakdown of fentanyl in the liver, which could decrease the therapeutic effects, e.g.
- rifampicin (antibiotic)
- carbamazepine, phenobarbital, phenytoin (for treatment of epilepsy)
Once you stop these medicines (CYP3A4 inducers) it may increase or prolong the therapeutic effects and side effects of fentanyl which can affect your breathing. In this situation you will require special monitoring and your dose will be adjusted.
You should not use Fencino® if you are taking Monoamine Oxidase Inhibitors (MAOI) (for treatment of depression or Parkinson's disease) or have taken them within the last 14 days.
Please tell your doctor if you are taking special medicines against depression, known as Serotonin Re-uptake Inhibitor (SSRI) or Serotonin Norepinephrine Re-uptake Inhibitor (SNRI) or a Monoamine Oxidase Inhibitor (MAOI). Your doctor should know any treatment with these medicines as the concomitant use with Fencino® may increase the risk for the potentially life-threatening serotonin syndrome.
If you are using Fencino® you should not take other pain killers like buprenorphine, nalbuphine or pentazocine because they could counteract the effects of fentanyl (e.g. analgesic effect) and may cause withdrawal symptoms in people who are dependent on opioids.
Fencino® with food, drink and alcohol
Patients, treated with Fencino® should not drink alcohol.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
There are no adequate data from the use of fentanyl, the active ingredient of Fencino®, in pregnant women. Therefore you should not use Fencino® during pregnancy unless your doctor decides it is necessary. There is a risk of neonatal withdrawal syndrome in newborn infants where the mother has used transdermal fentanyl a lot during pregnancy.
Use of Fencino® during childbirth is not recommended because fentanyl passes through the placenta and may cause breathing problems in the newborn child.
Breast-feeding
Fentanyl passes into the breast milk. Therefore breast-feeding should be stopped during treatment with Fencino® and for at least 72 hours after the removal of the patch.
Driving and using machines
Fencino® can influence the ability to drive and use machines.
In patients on a stable fentanyl dose, a significant impairment of the ability to drive and use machines is not expected. However at the beginning of treatment, upon increase of dose or upon combination with other medicinal products, some people's reactions may be affected, leading to impairment of the ability to drive or use machines. Such situations should be handled with caution.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
- The medicine has been prescribed to treat a medical or dental problem and
- You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
- It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Fencino® contains soya oil
If you are allergic to peanut or soya, do not use this medicinal product.
3. HOW TO USE FENCINO®
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
Adults
Determining the treatment amount (dosage adjustment)
Your doctor will decide which strength of Fencino® is the most appropriate for you. Your doctor will make the decision based upon:
- the intensity of your pain
- your general conditions
- your previous analgesic therapy
If you have not previously received strong analgesics, treatment should be initiated with the lowest concentration of the active substance.
Since the concentration of fentanyl in the bloodstream rises slowly, the current analgesic treatment should be reduced slowly until the desired analgesic effect is obtained. Fundamentally, the maximum analgesic effect can only be assessed after approximately 24 hours. Therefore, when switching from other strongly acting analgesics to Fencino®, the 24 hour requirement of the previously administered strong analgesic should first be calculated by your doctor.
Special populations
Elderly patients and those with hepatic or renal impairment should be observed carefully and the dose reduced if necessary.
Children aged 2 to 16 years old
Fencino® should only be prescribed to children aged 2 to 16, who have been already treated with at least 30 mg oral morphine doses per day or with any equivalent analgesic (morphine equivalent).
Your doctor will calculate the dosage of Fencino® as follows:
- For children who received 30 mg to 44 mg oral morphine equivalent per day the therapy should start with one Fencino® 12 pg/h transdermal patch.
- For children who received 45 mg to 134 mg oral morphine equivalent per day the therapy should start with two Fencino® 12 pg/h transdermal patches.
For children who received more than 90 mg oral morphine equivalent per day, only limited information is currently available with Fencino® 100 pg/h. If the analgesic effect of Fencino®
100 pg/h is insufficient, supplementary morphine or another short-duration opioid should be administered. Depending on the additional analgesic needs and the pain status of the child, your doctor may decide to increase the dose. Dose adjustments should be done in 12 pg/h (micrograms per hour) steps.
Adolescents aged 16 years and above
Adolescents aged 16 years and above please follow adult dosage.
Dosage for maintaining the effect
If the analgesic effect is still insufficient after 72 hours, the dose may be increased step by step until the desired analgesic effect is obtained. The required dose adjustments will be decided by your doctor. The possible need for other analgesics and the patient's sensation of pain should be taken into account.
Use of several patches simultaneously
If the required dose exceeds 100 microgram fentanyl per hour, several patches of differing strength may be used simultaneously.
Additional or alternative methods of analgesic treatment should be considered if the required dose exceeds 300 microgram fentanyl per hour.
|
Apply your patch on day |
Change your patch at the same time on |
|
Monday ^ |
Thursday |
|
Tuesday ^ |
Friday |
|
Wednesday ^ |
Saturday |
|
Thursday ^ |
Sunday |
|
Friday ^ |
Monday |
|
Saturday ^ |
Tuesday |
|
Sunday ^ |
Wednesday |
DE/H/1449:
|
Germany: |
Fentavera 12/25/50/75/100 Mikrogramm/Stunde transdermales Pflaster |
|
Spain: |
Fentanilo Matrix Acino 12/25/50/75/100 microgramos/hora parches transdermicos EFG |
|
United Kingdom: |
Fencino 12/25/50/75/100 micrograms/h transdermal patch |
DE/H/1519:
|
Germany: |
Fenylat 12/25/50/75/100 Mikrogramm/Stunde transdermales Pflaster |
|
Spain: |
Fenylat 12/25/50/75/100 microgramos/hora parche transdermico EFG |
|
France: |
Fentanyl Ranbaxy 12/25/50/75/100 microgrammes/heure, dispositif transdermique |
|
United Kingdom: |
Fenylat 12/25/50/75/100 micrograms/hour transdermal patch |
Change of treatment
If there is intent to switch from Fencino® to another strongly acting analgesic, the patch is removed, and the dosage of the new analgesic is adapted using the patient's pain assessment. After switching or reducing the dose, some patients may experience opioid withdrawal symptoms. If stoppage of Fencino® is necessary, step-by-step reduction of the dose is recommended.
How and when to use Fencino®
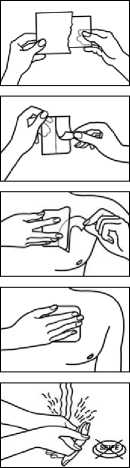
Immediately after the transdermal patch has been removed from the package and the release film as well as both sections of the backing film have been detached, the transdermal patch is attached to a hairless part of the skin or if this is not possible, hair at the application site should be clipped (not shaved) prior to application to the upper body (chest, back, upper arm). In young children, the upper back is the preferred location to apply the patch, to minimise the potential of the child removing the patch.
The skin should be cleaned carefully with clean water and thoroughly dried before the transdermal patch is attached (no cleaning agents should be used!). The transdermal patch is then adhered to the skin with the flat hand with a light pressure for 30 to 60 seconds. It must be ensured that the application area does not show any microlesions (e.g. due to radiation or shaving) or irritation.
As the outside of the transdermal patch is protected by a waterproof foil, the transdermal patch needs not be removed to take a shower.
No creams, oils, lotions or powders should be applied to the application area as they may impair the effective adhesion of the transdermal patch to the skin.
Only open the pouch immediately before use of the patch.
Advice for opening child resistant sachet:
1) You'll find a mark which shows you how to cut the sachet (using scissors!)
2) Rip the sachet alongside using the resulting cuts
3) Open the sachet and take out the patch
Then remove the pull-off foil from half of the patch. It is important to avoid touching the adhesive surface.
After sticking half of the patch onto the skin, you can remove the other half of the pull-off foil.
Firmly press the patch onto the skin with your flat hand for 30 - 60 seconds. Ensure that the adhesive edges stick well.
After applying the patch, wash your hands without using cleaning agents.
A patch that has been divided, cut or damaged in any way should not be used.
Always use this medicine exactly as your doctor has told you. Check with your doctor if you modify the treatment or discontinue.
How long to use Fencino®
You should change your patch after 72 hours (3 days). If required in individual cases, do not change the patch sooner than every 48 hours, since an increased risk of side effects (primarily respiratory suppression) must otherwise be expected. You must use a different skin area each time. Each used skin area can only be used again after at least 7 days. The analgesic effect may persist for some time after the transdermal patch has been removed.
If residue is found on the skin after the patch has been removed, remove it with soap and water. Do not use alcohol or other solvents, as these may penetrate the skin.
Please speak to your doctor if you have the impression that the effect of Fencino® is too strong or weak for you.
Using and changing the patches
• There is enough medicine in each patch to last 3 days (72 hours)
• You should change your patch every third day, unless your doctor has told you otherwise.
• Always remove the old patch before applying the new one.
• Always change your patch at the same time of day every 3 days (72 hours)
• Make a note of the day, date and time you apply a patch, to remind you when you need to change your patch.
• The following table shows you which day of the week to change your patch.
If you use more Fencino® than you should
If you have stuck on more patches than prescribed, remove the patches and contact your doctor or hospital for their opinion of the risk.
The most common sign of overdose is the reduced ability to breathe. Symptoms are that the person breathes too slowly or too weakly.
If this should occur, remove the patch and contact a doctor immediately. While waiting for the doctor, keep the person awake by talking to or shaking them now and then.
Other signs and symptoms of overdose are:
- drowsiness
- low body temperature
- slow heart rate
- decreased muscle tone
- deep sedation
- loss of muscle co-ordination
- constriction of the pupils
- convulsions
If you forget to use Fencino®
Do not under any circumstances use a double dose to make up a forgotten dose.
You should change your patch at the same time of day every three days (every 72 hours), unless directed otherwise by your doctor. If you forget, then change your patch as soon as you remember. If you are very late changing your patch then you should contact your doctor because you might need some extra painkillers.
If you stop using Fencino®
If you wish to interrupt or stop the treatment, you should always talk to your doctor.
Prolonged use of Fencino® can cause physical dependence. If you stop using the patches you may feel unwell.
As the risk of withdrawal symptoms is greater when the treatment is stopped suddenly, you should never stop treatment with Fencino® independently but always consult your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most serious undesirable effect of fentanyl is respiratory depression.
If this should occur, remove the patch and contact a doctor immediately. While waiting for the doctor, keep the person awake by talking to or shaking her/him now and then.
The following side effects are based on clinical trials with adults and children as well as experience of adults using this product after registration:
If any of the following side effects occur you should discontinue treatment and immediately contact your doctor or visit a hospital.
Very common: may affect more than 1 in 10 people
- sleepiness, dizziness, headache
- nausea, vomiting, constipation
Common: may affect up to 1 in 10 people
- hypersensitivity of the immune system
- loss of appetite
- not able to sleep, depression, anxiety, feeling confused, hallucinations
- tremor, pins and needles
- conjunctivitis (an eye infection)
- vertigo (feeling sick and dizzy)
- palpitations and an increase in heart rate
- increase in blood pressure
- shortness of breath
- diarrhoea, dry mouth, stomach pain, indigestion
- increased sweating, itching, rash, skin reddening
- muscle spasms
- difficulty passing urine
- tiredness, swelling, particularly in feet ankles and hands (peripheral oedema), rapid tiredness (asthenia), a feeling of discomfort and uneasiness (malaise), feeling cold
Uncommon: may affect up to 1 in 100 people
- agitation, disorientation, unnatural feeling of happiness,
- reduced sensation to touch (hypoaesthesia), convulsions (including clonic and Grand Mal seizures), memory gaps, speech disturbances, depressed level of consciousness, loss of consciousness
- vision blurred
- decreased heart rate, blue colouration of the skin (cyanosis)
- low blood pressure
- difficulties in breathing and breathlessness
- blockage of the intestine
- eczema, allergic rashes, skin disorders, contact dermatitis
- muscle twitching
- erectile dysfunction, sexual dysfunction
- application site reaction flu-like illness, feeling of body temperature change, application site hypersensitivity, withdrawal symptoms
- fever
Rare: may affect up to 1 in 1,000 people
- “pin-point” like pupils (myosis)
- irregular heartbeat (arrhythmia)
- flushing from dilation of blood vessels (vasodilatation)
- stopping breathing, shallow or too slow breathing (hypoventilation)
- partial blockage of the intestine
- skin inflammation and itching at the site where the patch was applied
Very rare: may affect up to 1 in 10,000 people
- painful gas
- less urine being passed and pain in the bladder
Not known: frequency cannot be estimated from the available data
- life threatening allergic reactions (anaphylactic shock), allergic reactions of different causes (anaphylactic or anaphylactoid reactions)
- slowed breathing (bradypnoea)
Other possible side effect
Tolerance of the medicine, leading to a physical dependence and psychological dependence can develop with repeated use of Fencino® (see section “Take special care with Fencino®”).
Opioid withdrawal symptoms (such as nausea, vomiting, diarrhoea, anxiety, and shivering) are possible in some patients after conversion from their previous opioid analgesic to Fencino® or if therapy is stopped suddenly (see section “Take special care with Fencino®”).
There have been very rare reports of newborn infants experiencing neonatal withdrawal syndrome when mothers used Fencino® a lot during pregnancy (see section “Take special care with Fencino®”).
In very rare cases soya oil may cause allergic reactions.
Additional side effects in children and adolescents
The side effects in children and adolescents treated with Fencino® transdermal patches is similar to the side effects observed in adults. Apart from side effects that can usually be expected during treatment of pain in severely ill children, no additional risks are known when Fencino® is given to children aged 2 years or older at 100 gg/hour as directed.
Very common side effects reported in the clinical trials performed in severely ill children were fever, headaches, vomiting, nausea, constipation, diarrhoea and itching.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE FENCINO®
Keep this medicine out of the sight and reach of children both before and after use.
Do not use this medicine after the expiry date which is stated on the label and outer packaging. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Disposal information
Used patches should be folded so that the adhesive side of the patch adheres to itself. The folded patch should be safely discarded. Accidental exposure to used and unused patches particularly in children may lead to a fatal outcome. Unused patches should be returned to the pharmacy.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Fencino® contains
The active substance is fentanyl.
Fencino 25 gg/h:
1 transdermal patch contains 5.1 mg fentanyl in a patch size of 8.5 cm2 and releases 25 micrograms fentanyl per hour.
The other ingredients are:
Matrix components: Aloe vera leaf extract oil (on the basis of soya oil tocopherol acetate), colophonium resin, poly(2-ethylhexylacrylate, vinylacetate) (50:50)
Release liner: Polyethylene terephtalate, polyester, siliconized Backing foil with imprint: polyethylene terephthalate foil, printing ink
What Fencino® looks like and contents of the pack
Transdermal patch.
Opaque, colourless, rectangular shaped patch with round corners and imprint on the backing foil: “Fentanyl 25gg/h” in single sealed sachets.
Fencino® is available in packs containing 5, 10 and 20 transdermal patches.
Marketing Authorisation Holder and Manufacturer:
Marketing Authorisation Holder: DB Ashbourne Ltd
The Rectory, Braybrooke Road Arthingworth, Market Harborough LE16 8JT, United Kingdom
Manufacturer: Acino AG
Am Windfeld 35, Miesbach
D-83714
Germany
This medicinal product is authorised in the Member States of the EEA under the
following names:
This leaflet was last revised in February 2016.
FEN25/0019/2
280mm x 480mm. Proof 09/12/15