Foradil
Out of date information, search anotherPlease read this leaflet carefully before you start to take
your medicine. It contains important information.
• Keep the leaflet in a safe place because you may want to read it again.
• If you have any other questions, or if there is something you don’t understand, please ask your doctor or pharmacist.
• This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Foradil®
(formoterol fumarate)
What you need to know about Foradil
Your doctor has decided that you need this medicine to help treat your condition.
Patient Information Leaflet
In this leaflet:
1. What Foradil is and what it’s used for
2. Things to consider before you start to take Foradil
3. How to take Foradil
4. Possible side effects
5. How to store Foradil
6. Further information
1. WHAT FORADIL IS AND WHAT IT’S USED FOR
The Foradil pack includes a blister pack of capsules containing the medicine and an inhaler device for taking the medicine.
Instructions for using the inhaler are given later on in the leaflet.
The active ingredient in this medicine is formoterol. Formoterol is one of a group of medicines called long-acting bronchodilators. It makes breathing easier by relaxing muscle spasms in the air passages of the lungs.
• Foradil is used to treat asthma in people who are already taking inhaled steroids but still have symptoms such as wheezing and breathlessness.
Taking Foradil regularly together with inhaled steroids will help to prevent breathing problems
• Foradil is also used to treat breathing problems in patients with chronic obstructive pulmonary disease (COPD)
2. THINGS TO CONSIDER BEFORE YOU START TO TAKE FORADIL
Some people MUST NOT take Foradil.
Talk to your doctor if:
• you know you’re allergic to Foradil, or if you have ever had an unusual or allergic reaction to formoterol or inhaled lactose
• you are breastfeeding
Foradil is not suitable for children under six years of age.
The very small amount of lactose in Foradil is unlikely to cause problems in lactose-intolerant patients.
You should also ask yourself these questions before taking Foradil. If the answer to any of these questions is YES, tell your doctor or pharmacist because Foradil might not be the right medicine for you.
• Is this the only asthma medicine you have?
• Do you suffer from any heart problems?
• Do you have high blood pressure?
• Do you have an overactive thyroid gland?
• Do you have an aneurysm (area where an artery is swollen because the wall of the artery is weak)?
• Do you have a heart disorder such as abnormal electrical signal called “prolongation of QT interval”?
• Are you diabetic?
• Do you have pheochromocytoma (a tumor of the adrenal gland that can affect blood pressure)?
• Are you pregnant?
Are you taking other medicines?
Some medicines can interfere with your treatment. Make sure your doctor knows if you are taking any of the following:
• Medicines for heart conditions including irregular heart beats, angina and heart failure
• Medicines for high blood pressure
• Beta blockers (either as tablets or eye drops)
• Water tablets (diuretics)
• Medicines for depression, for example, monoamine oxidase inhibitors (MAOIs) or tricyclics
• Anti-histamines or cold remedies (including any bought from the chemist)
• Medicines called ‘steroids’. Your doctor will know which these are
• Sympathomimetic agents, which are adrenaline-like medicines used to treat asthma and nasal congestion
• Phenothiazines, which are a group of medicines which control mental disorders such as schizophrenia, mania, psychotic conditions and anxiety
• Xanthines, for example theophylline or aminophylline, which are a class of medicines used to treat asthma and chronic obstructive airways diseases
• Macrolides (for example Erythromycin) used to treat bacterial infections.
• Anaesthetics like Halogenated hydrocarbons (for example Halothane). These drugs are used to produce anaesthesia during surgery.
• Anticholinergic drugs (for example Ipratropium bromide) used to treat gastrointestinal disorders and genitourinary disorders.
Always tell your doctor about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Other special warnings
• You must not use Foradil to treat a sudden asthma attack. Your doctor will have given you another inhaler (a ‘reliever’) for this
• It is very important to keep using your other asthma medicines, (inhaled steroids known as a ‘preventer’), regularly. DO NOT stop using them or change the dose when you start using Foradil
• If you feel that you are getting breathless or wheezy while you are using Foradil, you should continue to use it, but go to see your doctor as soon as possible in case you need another medicine
• Treatment with Foradil may lead to your blood level of potassium becoming too low. This may make you more susceptible to abnormal heart rhythm. Therefore, your doctor may monitor your blood level of potassium, especially if you have severe asthma
• Treatment with Foradil may lead to increased sugar levels in the blood. Therefore, you might need to monitor your blood sugar levels if you are diabetic
Will there be any problems with driving or using machinery?
In some patients, Foradil has been reported to cause dizziness. If you feel dizzy, do not drive or operate machinery.
3. HOW TO TAKE FORADIL
The doctor will tell you how much Foradil to take and when to take it. Always follow his/her instructions carefully. The dose will be on the pharmacist’s label. Check the label carefully. If you are not sure, ask your doctor or pharmacist. Keep using the inhaler for as long as you have been told unless you have any problems. In that case, check with your doctor. Foradil capsules must only be used with the inhaler provided. You must not try to inhale them using another inhaler. Also, do not put other types of capsules into the inhaler supplied with Foradil.
• Do not swallow the capsules. The contents of the capsule must be inhaled using the inhaler provided
For the treatment of asthma Adults and the elderly
• The usual dose is 1 puff (1 capsule=1 puff) morning and evening
• The usual dose for more severe cases is 2 puffs (2 capsules) morning and evening
• You should not take more than 4 capsules in a day Children aged 6 and over
• The usual dose is 1 puff (1 capsule=1 puff) morning and evening
• A child should not take more than 2 capsules in a day.
When you have asthma you must also be taking an inhaled steroid at the same time as Foradil.
For the treatment of COPD (Chronic Obstructive Pulmonary Disease)
Adults and the elderly
• The usual dose is 1 puff in the morning and evening
Children under 18
• Not applicable
The effects of this medicine will normally last for 12 hours. If you do get attacks of wheezing between doses, do not take extra doses of Foradil. Your doctor will have prescribed another medicine for this purpose. If you are not sure about this, check with your doctor.
• Do not stop or reduce the dose of Foradil or any other medicine for your breathing just because you feel better, without talking to your doctor first. It is very important to use these medicines regularly
• Do not increase your dose of Foradil without talking to your doctor first
• If you feel sick or very shaky or if you have an unusually fast heart beat, your Foradil dose may be too high. Tell your doctor as soon as possible
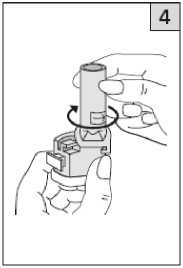
Twist the mouthpiece to the closed position until it clicks.
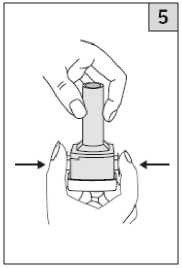
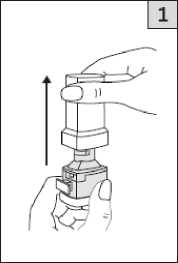
Pull off the cap.
Breathe out fully.
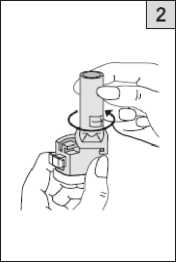
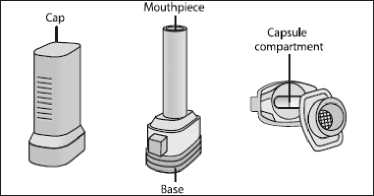
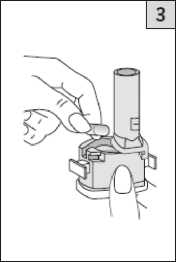
Take one of today’s capsules out of the blister strip. Place it in the capsuleshaped
compartment in the base of the inhaler. It is important that you remove the capsule from the blister pack only immediately before you use it.
POM
01923 332 796.
How to use the capsules with the inhaler device
A child should be shown how to use the inhaler correctly and should only use it with the help of an adult.
Keeping the inhaler upright, firmly squeeze the two blue buttons once only. This will pierce the capsule. Release the buttons. Although the capsule is now pierced, the powder will not be released until you inhale it.
Hold the base of the inhaler firmly and turn the mouthpiece in the direction of the arrow on the bottom of the mouthpiece to open.
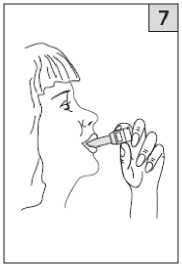
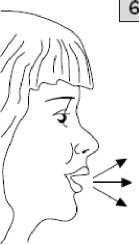
Place the mouthpiece in your mouth and tilt your head slightly
backwards. Close your lips around the mouthpiece and breathe in as quickly and as deeply as you can. As you breathe in, you will inhale the medicine into your lungs.
You should hear the capsule spinning in the inhaler. If you do not hear this whirring noise, the capsule may be stuck in the compartment. If this occurs, open the inhaler and loosen the capsule by prising it out of the compartment. Do not try to loosen the capsule by repeatedly pressing the buttons.
8. If you have heard the whirring noise, hold your breath for as long as you comfortably can while taking the inhaler out of your mouth. Then breathe normally. Open the inhaler to see if any powder is still in the capsule. If there is still powder in the capsule repeat steps 6 to 8.
9. After use, tip out the empty capsule and close the mouthpiece.
10. Replace the cap.
11. If you need to clean the inhaler, wipe the mouthpiece and capsule compartment with a dry cloth or a clean soft brush.
Very occasionally, very small pieces of the capsule shell can get in the mouth. If this happens, you may be able to feel these pieces on your tongue. The capsule shell is made of gelatin which is harmless to humans, and will soften or dissolve in the mouth and be swallowed. The chances of this happening will be increased if the capsule is pierced many times (Step 5); hence it is recommended that you do this only once for each capsule.
What if you forget to take a dose?
If you miss a dose, take it as soon as you remember. Then go on as before. Do not double the dose.
What if you take too much?
If you, or anyone else, accidentally takes too much, tell your doctor or your nearest hospital casualty department immediately.
Take your medicine pack with you so that people can see what you have taken.
4. POSSIBLE SIDE EFFECTS
Foradil is suitable for most people, but, like all medicines, it can sometimes cause side effects.
Some side effects can be serious
Stop using Foradil and tell your doctor straight away if you notice:
• Bronchospasm with wheezing or coughing and difficulty in breathing. This might affect up to 1 in 100 people.
• An allergic reaction such as if you feel faint (you might have low blood pressure), have a rash, or experience itching or facial swelling. This might affect less than 1 in 10,000 people.
• If you feel you have muscle weakness, muscle spasms or an abnormal heart rhythm (these could mean you have a low blood potassium level).
• If you feel you have an irregular heart beat or a fast heart beat. This might affect up to 1 in 10 people.
• If you feel a crushing chest pain (symptoms of angina pectoris). This might affect less than 1 in 1000 people.
The side effects listed below have also been reported.
Up to 1 in 10 people have experienced:
Headache, tremor, palpitations.
Up to 1 in 100 people have experienced:
Agitation, anxiety, feeling nervous, difficulties with sleeping, dizziness, fast heart beat, throat irritation, dry mouth, muscle cramps, muscle pain, worsening of asthma.
Very rare side effects (likely to affect less than 1 in 10,000 patients):
Nausea, distorted sense of taste, swelling of hands, ankles or feet, variations in blood pressure.
It is not known how frequently these effects may happen:
Excessive thirst, frequent urination and tiredness over an extended period of time (a possible indication of high blood sugar).
Headache and dizziness (symptoms of high blood pressure), cough and rash.
Some of these effects may wear off as you get used to the medicine.
If any of the symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor. He/she may want to give you a different medicine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE FORADIL
• Keep your medicine at room temperature in a dry place, below 25°C.
• Keep out of the sight and reach of children.
• Do not take the capsules out of the blister pack until you need them. Return any unused medicine to your pharmacist for safe disposal. Only keep it if your doctor tells you to.
• Do not take your capsules after the expiry date on the carton. Do not use the medicine after this date.
If your inhaler fails to work properly, or your capsules have become discoloured or show any other signs of deterioration, consult your pharmacist who will tell you what to do.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Foradil contains
The name of your medicine is Foradil. It comes as capsules containing a dry powder. The dry powder is inhaled into the lungs using the inhaler provided in the pack. Each capsule contains 12 micrograms of formoterol fumarate. Also contains the inactive ingredient lactose.
Each carton contains blister strips of capsules, in packs of 30 or 60 and a plastic inhaler device. The clear, hard gelatin capsules are marked ‘CG FXF’ and contain a dry white powder for inhalation.
The capsules are only for use with this inhaler and must not be swallowed.
Foradil is manufactured by Novartis Pharma AG, CH-4332, Switzerland and is procured from within the EU by the product licence holder: Caseview (PL) Limited, 20 Alliance Court, Alliance Road, London W3 0RB and repackaged by OPD Laboratories Ltd, Unit 6 Colonial Way, Watford, Herts WD24 4PR.
PL 13826/0357
Foradil®
Leaflet revision date (ref) 10/01/2013.
To request a copy of this in Braille, large print or audio please call
Foradil is a registered Trademark of Novartis Pharmaceuticals UK Ltd.