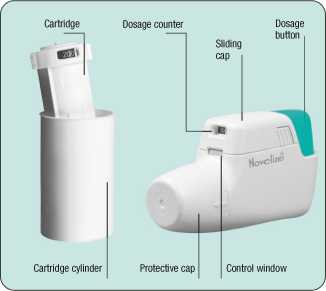
Formatris Novolizer 12 Micrograms Per Actuation Inhalation Powder
1. PREPARATION:
The NOVOLIZER is a breath actuated Dry Powder Inhaler makes inhaling simple and reliable. Its straightforward use, fast cartridge replacement and simple cleaning are easy and quickly done.
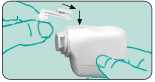
Place the NOVOLIZER Dry Powder Inhaler in front of you. Lightly press together the ribbed surfaces on both sides of the cap, move the cap forwards (^) and lift off (t).
Remove the protective aluminium foil from the cartridge cylinder and take out the new car-tridge.This should be done just before using the cartridge. The colour coding on the cartridge must correspond to the colour of the dosage button.
The small red disc on the bottom of the cartridge cylinder is a drying agent which should be disposed of after removal of the cartridge. First filling:
Insert the cartridge into the NOVOLIZER Dry Powder Inhaler with the dosage counter facing the mouthpiece (4). Do not press the dosage button while inserting the cartridge.
Refilling:
Note: The NOVOLIZER Dry Powder Inhaler should be cleaned every time the cartridge is exchanged after removal of empty cartridge.
If you have already used the NOVOLIZER Dry Powder Inhaler, first remove the empty cartridge and then insert the new one (4). Do not press the dosage button while inserting the cartridge.
Replace the cap into the side guides from above (4) and push down flat towards the coloured dosage button until it snaps into place (^).
The NOVOLIZER is now filled and ready for use.
You can leave the cartridge in the NOVOLIZER dry powder inhaler until it is used up or for up to 6 months after insertion. Remember to replace the cartridge every 6 months even if it is not empty. The cartridge is used up if you see a hatched “0” in the middle of the control window. Then a new cartridge has to be inserted. The cartridges can only be used in the original Novolizer dry powder inhaler.
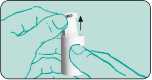

4. Check that the colour of the control window has changed back to red, which also indicates that you have breathed in strongly enough and correctly. Replace the protective cap on the mouthpiece - the inhalation procedure is now complete.
The number in the top window indicates the number of inhalations left. The numeric scale 60-0 is shown in steps of 10. If the click sound and change of the colour did not appear, please repeat the procedure as described above. When “0” appears in the middle of the counter window the cartridge must be replaced with a new one.
NOTE: The coloured dosage button should only be pressed immediately before breathing in. Taking a double dose or taking more than one dose at a time is not possible with the NOVOLIZER. The click sound and the change of the colour in the control window indicate that you have breathed in strongly enough and correctly. If the colour of the control window does not change back to red, the inhalation (puff) should be repeated. If inhalation is not completed correctly after several attempts, then you should consult your doctor.
3. CLEANING:
The NOVOLIZER Dry Powder Inhaler should be cleaned at regular intervals, but at least every time the cartridge is changed.
Remove protective cap and mouthpiece First remove the protective cap. Then grasp the mouthpiece and turn it briefly counterclockwise (t) until it becomes loose. Then remove (^).
Cleaning
Now turn the NOVOLIZER upside down. Grasp the loose dispensing slide and move it forwards (<-)
and upwards (t). Any remaining powder can be removed by tapping lightly.
Clean the mouthpiece, the dispensing slide and the powder inhaler with a soft and dry lint-free cloth.
Do NOT use water or detergent.
Assembly - Insert dispensing slide After cleaning insert the dispensing slide by sliding down at an angle (() and press into position (4).
Turn the inhaler back over.

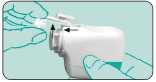
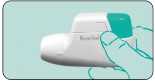
Assembly - Fit mouthpiece and protective cap Insert the mouthpiece r— with the pin into the groove on the left and turn to the right until it snaps into place. Finish by replacing the protective cap.
Package leaflet: Information for the patient
Formatris Novolizer®
12 micrograms per actuation inhalation powder
(formoterol)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- I f you have further questions, please ask your doctor or your pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
2. USAGE:
1. Whenever possible, sit or stand while inhaling. When using the NOVOLIZER always keep it horizontal. First remove the protective cap (^

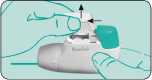
2. Completely depress the coloured dosage button. A loud double click will be heard and the colour of the control window will change from red to green. Then release the coloured button. The colour green in the control window indicates that the NOVOLIZER is ready for use.
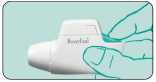
3. Breathe out deeply (but not into the NOVOLIZER Dry Powder Inhaler).
Put your lips tightly around the mouthpiece, inhale the powder with a deep breath, during this breath a loud click should be heard, which indicates that you have breathed in strongly enough and correctly. Then hold the breath for a few seconds and continue to breathe out slowly through the nose. If you need to take another dose repeat steps 2 and 3.

Notes
• The Patient Information Leaflet describes how the drug works. Please read it through carefully before using the inhaler for the first time.
• The NOVOLIZER which comes with various active substances does not use any propellants and is designed for refilling. This makes the NOVOLIZER a very environmentally friendly product.
• It is not possible to overdose with the NOVOLIZER. Even if the button is pressed several times, no more powder is available for inhalation. Only press the button when you really want to inhale. If you cannot manage to inhale correctly after several attempts, consult your doctor.
• The NOVOLIZER can be refilled using new cartridges* containing the active substance and is thus ideally suited to long-term usage (up to one year).
• Do not shake the filled NOVOLIZER.
• Please support your children in proper handling of the device.
• Make sure your NOVOLIZER is protected from moisture and heat and kept clean at all times.
*For information on other medicines which
can be used in the Novolizer dry powder
inhaler, please contact your doctor.
MEDA Pharma GmbH & Co. KG
Benzstr. 1,61352 Bad Homburg (Germany)
Last Review February 2014
O
LO
What is in this leaflet:
1. What Formatris Novolizer inhalation powder is and what it is used for
2. What you need to know before you use Formatris Novolizer inhalation powder
3. How to use Formatris Novolizer inhalation powder
4. Possible side effects
5. How to store Formatris Novolizer inhalation powder
6. Contents of the pack and other information
1. WHAT FORMATRIS NOVOLIZER INHALATION POWDER IS AND WHAT IT IS USED FOR
Formatris Novolizer is a powder for inhalation containing formoterol. Formoterol belongs to a group of medicines known as long-acting B2 agonists. B2 agonists relax the muscles in the airways in your lungs and by doing this open up your airways so that you can breathe more easily. They give long-lasting relief (up to 1 2 hours) of symptoms such as wheezing, shortness of breath and coughing.
Formoterol is used to treat patients with asthma already being treated with inhaled corticosteroids (also known simply as steroids). Inhaled corticosteroid and formoterol taken together are long-term treatments for asthma and must be taken regularly every day to prevent symptoms of asthma occurring. Such as increasing shortness of breath, wheezing, coughing.
Formatris Novolizer is also used for the relief of symptoms such as wheezing, shortness of breath and coughing in patients with chronic obstructive pulmonary disease (COPD).
2. WHAT YOU NEED TO KNOW BEFORE YOU USE FORMATRIS NOVOLIZER INHALATION POWDER
Do NOT use Formatris Novolizer if you
are allergic (hypersensitive) to formoterol or the other ingredient of this medicine listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Formatris Novolizer.
Formatris Novolizer should not be used (and is not sufficient) as the first treatment for asthma.
Formatris Novolizer should not be used to treat an acute asthma attack or for relief of acute symptoms of asthma. In these situations you should take your short-acting bronchodilator, for example your salbuta-mol inhaler, also known as your blue “reliever” inhaler, to treat an acute asthma attack and you should contact your doctor or asthma nurse straightaway.
If you are an asthmatic patient, you should also be receiveing treatment with a corticosteroid (an anti-inflammatory treatment for your asthma). Formatris Novolizer must always be prescribed together with a corticosteroid inhaler and it is important that you continue taking the steroid inhaler while you are taking Formatris Novolizer inhalation powder. You should not decrease the dose of your steroid inhaler when you start taking Formatris Novolizer inhalation powder without medical advice even when your symptoms improve.
If you need to take an extra treatment, e.g. your blue “reliever” to prevent exercise-induced asthma symptoms more frequently than you would usually do so, or several times a week, this may be a sign that your asthma control is not good enough. Therefore you should contact your doctor or asthma nurse so that your asthma and your treatment can be reassessed.
Take special care and talk to your doctor before using Formatris Novolizer if you have
• any heart disease,
• blood pressure problems,
• diabetes,
• an overactive thyroid gland e.g. thyrotoxicosis or
• problems with adrenal glands e.g. pha-eochromocytoma.
If any of the above apply to you, you must tell your doctor. He/she may wish to alter your treatment or monitor your condition more closely.
As with other inhaled medicines there is a risk of paradoxical bronchospasm (acute wheezing or shortness of breath or a feeling of tightness in the chest or severe coughing immediately after using the Formatris Novolizer). If this occurs do not take any more puffs from the Formatris Novolizer. You should talk immediately with your doctor.
Should your symptoms continue or worsen or the number of doses of formoterol you need to take to control symptoms increases, this usually indicates a worsening of disease, In these situations you should contact your doctor or your asthma nurse in order that your asthma and your treatment can be re-assessed.
Children
This medicine is not recommended for use in children below the age of 6.
Other medicines and Formatris Novolizer
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other inhalers or other medicines.
In particular, this applies for:
• other B2agonists also known as broncho-dilators e.g. salbutamol, salmeterol or ephedrine
• xanthine derivatives used for the treatment of asthma (e.g. theophylline)
• steroid medicines (e.g. beclomethasone dipropionate, fluticasone propionate, and steroid tablets eg, prednisolone)
• diuretics or water tablets (e.g., furose-mide)
• antihistamines (e.g. terfenadine, astem-izole)
• digoxin used to treat heart disease.
• medicines for a rapid or irregular heart beat (e.g. quinidine, disopyramide, procainamide)
• phenothiazines (e.g. chlorpromazine) used to treat mental illness or severe nausea (feeling sick) and vomiting (being sick)
• antibiotics (e.g. erythromycin)
• tricyclic antidepressants (e.g. amitriptyline)
• monoamine oxidade inhibitors taken within the last 14 days (e.g. phenelzine)
• hormones (e.g. L-thyroxine, oxytocin)
• beta-blockers for high blood pressure or angina (taken by mouth as e.g. atenolol, metoprolol or as eye drops e.g. timolol)
• Some general anaesthetics may interact with formoterol to cause irregular heart rhythms and a fall in blood pressure. Therefore, if you are having an operation, advise hospital staff that you are taking Formatris Novolizer.
• As with other medicines of this type, if formoterol is taken with alcohol, or with medicines for Parkinson’s disease or thyroid problems you may experience an increase in heart rate (pulse).
Formatris Novolizer with food, drink and alcohol
You should avoid drinking of alcohol because you may experience an increase
cd
D
03
c
CT
£
-C
u
<D
c
=)
cd
03
T3
E
D
CD
<D
CD
-Q
03
'CD
□
03
E
<D
U)
CD
E
in in
in vd o o \d co in o
Cl Cl fN
03
E
o o o o o o o o
Q. Q. Q. Q.
rn in \£>

C
*
I
-Q
E
u
u\
c
3
u
£
3
C
(0
§
m
(N
co
I
I
5 S.E S
cS r-» z .Q

1
V)
-t-1
c
O
CD
"O
03

=5 U
CO
-O
CD
§
<N
V) <3-‘Z rs
o
% a
O 03
£ S
E
E
o
CO
T5006274_p02.indd 1
03.06.15 15:16
in heart rate (pulse). Food and other drinks are not known to alter the effectiveness of Formatris Novolizer.
Pregnancy and breast-feeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Formoterol should only be used during pregnancy (particularly during the first three months and at the end of the pregnancy and during labour), after careful consideration and if there are no other safer alternatives.
It is not known whether formoterol passes into human breast milk. Therefore, administration of Formatris Novolizer during breastfeeding should only be considered if the expected benefit to you is greater than any possible risk to the child.
Driving and using machines
Formatris Novolizer does not affect the ability to drive or use machines.
Formatris Novolizer contains milk sugar (lactose)
Normally, the content of lactose in a single dose does not cause any problems in people with lactose intolerance. Milk sugar (lactose) may contain small amounts of milk protein.
Always use this medicine exactly as your doctor or asthma nurse has told you. Check with your doctor or pharmacist if you are not sure. The label should remind you of how many actuations (puffs) to take and how often you should take them. Do not take more than your doctor or asthma nurse told you to. Formatris Novolizer is for inhalation use. It should be inhaled through your mouth into your lungs. If the person taking this medicine is a child, treatment should be supervised by an adult. The child should be shown how to use the inhaler correctly and may need the help of an adult to use the inhaler.Please read the instructions for using this device carefully before taking this medicine.
The recommended dose is:
Asthma:
Adults (including older people) and adolescents over 12 years of age:
The usual dose for adults is 1 puff in the morning and 1 puff again in the evening. For more severe asthma your doctor may prescribe 2 puffs twice daily.
The maximum daily dose is 4 puffs (2 puffs inhaled twice daily).
Use in children 6 years and older:
The usual dose for children is 1 puff in the morning and 1 puff again in the evening. For more severe asthma your doctor may prescribe 2 puffs twice daily.
The maximum daily dose is 4 puffs (2 puffs inhaled twice daily).
The regular daily dose should not exceed 2 puffs, however, occasionally up to a maximum of 4 puffs may be allowed within a 24-hours period.
Use in children below 6 years of age:
Formatris Novolizer is not recommended for use in children under the age of 6 years as insufficient experience is available in this age group.
COPD (chronic obstructive pulmonary disease):
Adults (including older people) and adolescents over 12 years of age:
The usual dose is 1 puff in the morning and 1 puff again at night.
The daily dose for regular use should not exceed 2 puffs.
If necessary, additional puffs above those prescribed for regular therapy may be used for relief of symptoms, up to a maximum total daily dose of 4 puffs. More than 2 puffs should not be taken on any single occasion.
For inhalation use. The powder should be inhaled through your mouth into your lungs. Inhale as shown in the ‘Instructions for Use’ on the back of this leaflet.
If you use more Formatris Novolizer than you should
The symptoms of an overdose are the same as the more common side effects
and might include tremor, headache, palpitations, feeling sick.
If any of these reactions occur you should contact your doctor immediately.
If you forget to use Formatris Novolizer inhalation powder
Take your dose as soon as you remember, if it is nearly time for the next dose just take the next dose at the usual time.
Do not take a double dose to make up for a forgotten dose.
Do not stop or reduce the dose of For-matris Novolizer or any other medicine for your breathing just because you feel better, without talking to your doctor first. It is very important to use these medicines regularly.
If you feel sick or very shaky or if you have an unusually fast heart beat, your Forma-tris Novolizer dose may be too high. Tell your doctor as soon as possible.
Once your symptoms are controlled your doctor may decide to reduce the dose of Formatris Novolizer inhalation powder gradually so that your asthma is treated with the lowest effective dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects are listed below according to their frequency. If you are not sure what the side effects below are, ask your doctor to explain them to you.
Most important side effects
• as with all inhalers it is possible for the powder to make you wheezy immediately after inhalation. This occurs rarely (may affect up to 1 in 1,000 people).
• rarely an allergic reaction may result in a condition known as angioneurotic oedema which is a swelling of the face, lips, eyes, and throat. If any of these allergic reactions occur you must stop taking Formatris Novolizer immediately and contact your doctor.
• very rarely (may affect up to 1 in 10,000 people) severe chest pain (angina pectoris) can occur
List of all other side effects:
Common side effects (may affect up to 1 in 10 people):
Headache, tremor and palpitations-fast and sometimes irregular heart beat.
Uncommon side effects (may affect up to 1 in 100 people):
Agitation, restlessness, difficulty sleeping, muscle cramps and irritation of the throat. Rare side effects (may affect up to 1 in
1.000 people):
Rapid or irregular heart beat, nausea (feeling sick), changes in potassium levels that may cause muscle weakness, nervousness and wheeziness.
Very rare side effects (may affect up to 1 in
10.000 people):
Increased risk of severe heart rhythm abnormality in sensitive patients, hypergly-caemia (increase in blood glucose), unusual taste, dizziness, and changes in blood pressure
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/ yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE FORMATRIS NOVOLIZER INHALATION POWDER
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label, the carton and the cartridge container. The expiry date refers to the last day of that month.
Storage conditions
Store the cartridge in the original package until required for use.
After opening the cartridge container and inserting into the Novolizer device, store
Formatris Novolizer in a dry place protected from moisture and below 25 °C.
Information concerning the shelf-life after first opening
Exchange the cartridge 6 months after first opening.
Do not use the powder inhaler for more than a year.
Note: The Novolizer device should be replaced after 2000 inhalations (puffs). Therefore, a maximum of 33 cartridges containing 60 single doses should be used with this device (within a single year).
The Novolizer should be cleaned at regular intervals, but at least every time the cartridge is changed. Instructions on how to clean the device can be found in the instructions for use on the back of this leaflet.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Formatris Novolizer 12 micrograms per actuation inhalation powder contains
Formatris Novolizer 12 micrograms per actuation inhalation powder is a dry powder for inhalation. The active substance is formoterol.
One puff contains 8.36 micrograms for-moterol (as fumarate dihydrate).
The other ingredient is lactose monohydrate (milk sugar). Formatris Novolizer 12 micrograms per actuation inhalation powder contains 11.488 mg of lactose per puff.
What Formatris Novolizer looks like and contents of the pack
Formatris Novolizer inhalation powder, contains a white powder in a cartridge and is available in the following packs:
Original sales packs:
1 powder inhaler and 1 cartridge with at least 60 puffs
1 powder inhaler and 2 cartridges with at least 60 puffs each
Refill packs:
1 cartridge with at least 60 puffs
2 cartridges with at least 60 puffs each
3 cartridges with at least 60 puffs each
Not all pack sizes may be marketed.
Marketing Authorization Holder
MEDA Pharmaceuticals Ltd Skyway House Parsonage Road Takeley
Bishop’s Stortford CM22 6PU United Kingdom
VEMEDIA Manufacturing B.V.
Verrijn Stuartweg 60,
NL-1112 AX Diemen,
The Netherlands
(alternative:
MEDA Manufacturing GmbH Neurather Ring 1 D-51063 Cologne Tel: (+49)-221-6472-0 Fax: (+49)-221-6472-696
Alternative:
MEDA Pharma GmBH & Co. KG
BenstraBe 1
D-61352 Bad Homburg
Tel: (+49)-6172-888-01
Fax: (+49)-6172-888-2740
This medicinal product is authorized in the Member States of the EEA under the following names:
Austria:
Novolizer Formoterol Meda 12 Mikrogramm Pulver zur Inhalation
Belgium and Luxembourg:
Novolizer Formoterol 12 microgrammes, poudre pour inhalation
Germany:
Formoterol Novolizer 12 Mikrogramm, Pulver zur Inhalation
France:
Asmelor Novolizer 12 microgrammes/dose, poudre pour inhalation
Ireland:
Novolizer Formoterol 12 micrograms/dose inhalation powder
Italy:
Formoterolo ViatrisNovolizer 12 micro-grammi/dose polvere per inalazione
Netherlands:
Formoterol Novolizer 12 microgram, inha-latiepoeder
Portugal:
Formoterol Novolizer 12 microgramas/dose, po para inalagao
Spain:
Formatris Novolizer 12 microgramos/dosis, polvo para inhalacion
This leaflet was last revised in February 2014
Asthma can be controlled in different ways. Your doctor will work with you to decide on a treatment plan which best suits your individual needs and which should help to reduce your asthma symptoms and the number of asthma attacks.
There are also ways that you can help yourself, such as:
• Try to avoid or reduce contact with things that may trigger an asthma episode, for example, animal fur, smoking (including passive smoking), moulds, pollen and house dust mites.
• If you know that exercise is a trigger for your asthma, make sure that you have followed your doctor’s instructions before starting any exercise.
• Be honest with your doctor or asthma nurse about how asthma affects you day-to-day and develop with them a treatment plan that suits your particular lifestyle. Remember to take your asthma medication as your doctor has instructed.
• If you have any worries about your medication, discuss this with your local pharmacist, asthma nurse or doctor.
jy
O
03
C
05
'oi
U
o
c
D
&
03
~o
£
O
03
o
05
<V
<V
_Q
03
Of
'<u
rtS
£
(V
05
a3
£
<v
CD
L/5 L/5
Ir> VO © © vd ro <n ©
to
£
re
CL CL CL CL CL CL
<— CN CO LO VO

I
-Q
o>
"O
0 u
1
‘oo
c
o
oo
3
O
fl3

<D
U
<D
05
£
u
©
oo
=«= <N 03
T5006274_p02.indd 2
03.06.15 15:16