Furosemide 40mg Tablets
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
Furosemide 40mg Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains Furosemide 40mg.
For a full list of excipients, see section 6.1.
3
PHARMACEUTICAL FORM
Tablet
White-to off white, flat, uncoated tablets with beveled edges, debossed
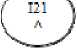
on one side and breakline on the other side
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Furosemide is a potent diuretic with rapid action.
Furosemide tablets are indicated for:
• The treatment of fluid retention associated with heart failure, including left ventricular failure, cirrhosis of the liver and renal disease, including nephrotic syndrome.
• The treatment of mild to moderate hypertension when brisk diuretic response is required. Alone or in combination with other antihypertensive agents in the treatment of more severe cases.
4.2. Posology and method of administration
For oral administration.
Adults: The initial adult dose is 40mg daily, reduced to 20mg daily or 40mg on alternative days. In some patients daily doses of 80mg or higher (given in divided doses) may be required.
Elderly: Caution is advised as furosemide is excreted more slowly in the elderly. Treatment should be started with 20mg and titrated upwards as required (see section 4.4).
Children: Contra-indicated (see section 4.3)
4.3. Contraindications
- Hypersensitivity to furosemide, amiloride, sulphonamides or sulphonamide derivatives, and/or any of the excipients of the product.
- Hypovolaemia and dehydration (with or without accompanying hypotension) (see section 4.4)
- Severe hypokalaemia: severe hyponatraemia (see section 4.4).
- Comatose or pre-comatose states associated with hepatic cirrhosis (see section 4.4).
- Anuria or renal failure with anuria not responding to furosemide, renal failure as a result of poisoning by nephrotoxic or hepatotoxic agents or renal failure associated with hepatic coma
- Impaired renal function with a creatinine clearance below 30ml/min per 1.73 m2 body surface area (see section 4.4).
- Addison’s disease (see section 4.4).
- Children and adolescents under 18 years of age (safety in this age group has not yet been established).
- Digitalis intoxication (see section 4.5).
- Concomitant potassium supplements or potassium sparing diuretics (see section 4.5).
- Porphyria
- Breast-feeding women (see section 4.6).
4.4. Special warnings and precautions for use
Conditions requiring correction before furosemide is started (see also section 4.3)
• Hypotension.
• Hypovolaemia.
• Severe electrolyte disturbances - particularly hypokalaemia, hyponatraemia and acid-base disturbances.
Furosemide is not recommended
• In patients at high risk for radiocontrast nephropathy - it should not be used for diuresis as part of the preventative measures against radiocontrast-induced nephropathy.
• In patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption.
Particular caution and/or dose reduction required:
• elderly patients (lower initial dose as particularly susceptible to side-effects - see section 4.2)
• difficulty with micturition including prostatic hypertrophy (increased risk of urinary retention: consider lower dose). Closely monitor patients with partial occlusion of the urinary tract
• diabetes mellitus (latent diabetes may become overt: insulin requirements in established diabetes may increase: stop furosemide before a glucose tolerance test)
• pregnancy (see section 4.6)
• gout (furosemide may raise uric acid levels/precipitate gout)
• patients with hepatorenal syndrome
• impaired hepatic function (see section 4.3 and below - monitoring required)
• impaired renal function (see section 4.3 and below - monitoring required)
• adrenal disease (see section 4.3 - contraindication in Addison’s disease)
• hypoproteinaemia e.g. nephrotic syndrome (effect of furosemide may be impaired and its ototoxicity potentiated - cautious dose titration required).
• acute hypercalcaemia (dehydration results from vomiting and diuresis - correct before giving furosemide). Treatment of hypercalcaemia with a high dose of furosemide results in fluid and electrolyte depletion - meticulous fluid replacement and correction of electrolyte required.
• Patients who are at risk from a pronounced fall in blood pressure
• premature infants (possible development nephrocalcinosis/nephrolithiasis; renal function must be monitored and renal ultrasonography performed).
Avoidance with other medicines (see also section 4.5 for other interactions)
• concurrent NSAIDs should be avoided - if not possible diuretic effect of furosemide may be attenuated
• ACE-inhibitors & Angiotensin II receptor antagonists - severe hypotension may occur - dose of furosemide should be reduced/stopped (3 days) before starting or increasing the dose of these
Laboratory monitoring requirements:
• Serum sodium
Particularly in the elderly or in patients liable to electrolyte deficiency
• Serum potassium
The possibility of hypokalaemia should be taken into account, in particular in patients with cirrhosis of the liver, those receiving concomitant treatment with corticosteroids, those with an unbalanced diet and those who abuse laxatives. Regular monitoring of the potassium, and if necessary treatment with a potassium supplement, is recommended in all cases, but is essential at higher doses and in patients with impaired renal function. It is especially important in the event of concomitant treatment with digoxin, as potassium deficiency can trigger or exacerbate the symptoms of digitalis intoxication (see section 4.5). A potassium-rich diet is recommended during long-term use.
Frequent checks of the serum potassium are necessary in patients with impaired renal function and creatinine clearance below 60ml/min per 1.73m2 body surface area as well as in cases where furosemide is taken in combination with certain other drugs which may lead to an increase in potassium levels (see section 4.5 & refer to section 4.8 for details of electrolyte and metabolic abnormalities) • Renal function
Frequent BUN in first few months of treatment, periodically thereafter. Long-term/high-dose BUN should regularly be measured. Marked diuresis can cause reversible impairment of kidney function in patients with renal dysfunction. Adequate fluid intake is necessary in such patients. Serum creatinine and urea levels tend to rise during treatment
• Glucose
Adverse effect on carbohydrate metabolism - exacerbation of existing carbohydrate intolerance or diabetes mellitus. Regular monitoring of blood glucose levels is desirable.
• Other electrolytes
Patients with hepatic failure/alcoholic cirrhosis are particularly at risk of hypomagnesia (as well as hypokalaemia). During long-term therapy (especially at high doses) magnesium, calcium, chloride, bicarbonate and uric acid should be regularly measured.
Clinical monitoring requirements (see also section 4.8):
Regular monitoring for
• blood dyscrasias. If these occur, stop furosemide immediately
• liver damage
• idiosyncratic reactions
Other alterations in lab values
• Serum cholesterol and triglycerides may rise but usually return to normal within 6 months of starting furosemide
Concomitant use with risperidone
In risperidone placebo-controlled trials in elderly patients with dementia, a higher incidence of mortality was observed in patients treated with furosemide plus risperidone (7.3%; mean age 89 years, range 75-97 years) when compared to patients treated with risperidone alone (3.1%; mean age 84 years, range 70-96 years) or furosemide alone (4.1%; mean age 80 years, range 67-90 years). Concomitant use of risperidone with other diuretics (mainly thiazide diuretics used in low dose) was not associated with similar findings.
No pathophysiological mechanism has been identified to explain this finding, and no consistent pattern for cause of death observed. Nevertheless, caution should be exercised and the risks and benefits of this combination or co-treatment with other potent diuretics should be considered prior to the decision to use. There was no increased incidence of mortality among patients taking other diuretics as concomitant treatment with risperidone. Irrespective of treatment, dehydration was an overall risk factor for mortality and should therefore be avoided in elderly patients with dementia (see section 4.3 Contraindications).
4.5. Interaction with other medicinal products and other forms of interaction
General- The dosage of concurrently administered cardiac glycosides, diuretics, antihypertensive agents, or other drugs with blood-pressure-lowering potential may require adjustment as a more pronounced fall in blood pressure must be anticipated if given concomitantly with furosemide.
The toxic effects of nephrotoxic drugs may be increased by concomitant administration of potent diuretics such as furosemide.
Some electrolyte disturbances (e.g. hypokalaemia, hypomagnesaemia) may increase the toxicity of certain other drugs (e.g. digitalis preparations and drugs inducing QT interval prolongation syndrome).
Antihypertensives - enhanced hypotensive effect possible with all types. Concurrent use with ACE inhibitors or Angiotensin II receptor antagonists can result in marked falls in blood pressure, furosemide should be stopped or the dose reduced before starting an ACE-inhibitor or Angiotensin II receptor antagonists (see section 4.4)
Antipsychotics - furosemide-induced hypokalaemia increases the risk of cardiac toxicity. Avoid concurrent use with pimozide. Increased risk of ventricular arrhythmias with amisulpride or sertindole. Enhanced hypotensive effect with phenothiazines.
When administering risperidone, caution should be exercised and the risks and benefits of the combination or co-treatment with furosemide or with other potent diuretics should be considered prior to the decision to use. See section 4.4 Special warnings and precautions for use regarding increased mortality in elderly patients with dementia concomitantly receiving risperidone.
Anti-arrhythmics (including amiodarone, disopyramide, flecanaide and sotalol) - risk of cardiac toxicity (because of furosemide-induced hypokalaemia). The effects of lidocaine, tocainide or mexiletine may be antagonised by furosemide.
Cardiac glycosides - hypokalaemia and electrolyte disturbances (including hypomagnesia) increase the risk of cardiac toxicity.
Drugs that prolong Q-T interval - increased risk of toxicity with furosemide-induced electrolyte disturbances
Vasodilators - enhanced hypotensive effect with moxisylyte (thymoxamine) or hydralazine
Other diuretics - profound diuresis possible when furosemide given with metolazone. Increased risk of hypokalaemia with thiazides. Contraindicated with potassium sparing diuretics (eg Amiloride spironolactone) - increased risk of hyperkalaemia (see section 4.3)
Renin inhibitors - aliskiren reduces plasma concentrations of furosemide Nitrates - enhanced hypotensive effect
Lithium - In common with other diuretics, serum lithium levels may be increased when lithium is given concomitantly with furosemide, resulting in increased lithium toxicity, including increased risk of cardiotoxic and neurotoxic effects of lithium.
Therefore, it is recommended that lithium levels are carefully monitored and where necessary the lithium dosage is adjusted in patients receiving this combination.
Chelating agents - sucralfate may decrease the gastro-intestinal absorption of furosemide - the 2 drugs should be taken at least 2 hours apart
NSAIDs - increased risk of nephrotoxicity. Indometacin and ketorolac may antagonise the effects of furosemide (avoid if possible see section 4.4)
Salicylates - effects may be potentiated by furosemide. Salycylic toxicity may be increased by furosemide
Antibiotics - increased risk of ototoxicity with aminoglycosides, polymixins or vancomycin - only use concurrently if compelling reasons. Increased risk of nephrotoxicity with aminoglycosides or cefaloridine. Furosemide can decrease vancomycin serum levels after cardiac surgery. Increased risk of hyponatraemia with trimethoprim. Impairment of renal function may develop in patients receiving concurrent treatment with furosemide and high doses of certain cephalosporins.
Antidepressants - enhanced hypotensive effect with MAOIs. Increased risk of postural hypotension with TCAs (tricyclic antidepressants). Increased risk of hypokalaemia with reboxetine
Antidiabetics - hypoglycaemic effects antagonised by furosemide
Antiepileptics - increased risk of hyponatraemia with carbamazepine. Diuretic effect reduced by phenytoin.
Antihistamines - hypokalaemia with increased risk of cardiac toxicity
Antifungals - increased risk of hypokalaemia and nephrotoxicity with amphoterecin
Anxiolytics and hypnotics - enhanced hypotensive effect. Chloral or triclorfos may displace thyroid hormone from binding site.
CNS stimulants (drugs used for ADHD) - hypokalaemia increases the risk of ventricular arrhythmias
Corticosteroids - diuretic effect anatgonised (sodium retention) and increased risk of hypokalaemia
Glychyrrizin -(contained in liquorice) may increase the risk of developing hypokalaemia.
Carbenoxolone -may increase the risk of developing hypokalaemia
Cytotoxics - increased risk of nephrotoxicity and ototoxicity with platinum compounds/cisplatin. Nephrotoxicity of cisplatin may be enhanced if furosemide is not given in low doses (e.g. 40 mg in patients with normal renal function) and with positive fluid balance when used to achieve forced diuresis during cisplatin treatment.
Anti-metabolites - effects of furosemide may be reduced by methotrexate and furosemide may reduce renal clearance of methotrexate
Potassium salts - contraindicated - increased risk of hyperkalaemia (see section 4.3) Dopaminergics - enhanced hypotensive effect with levodopa.
Immunomodulators - enhanced hypotensive effect with aldesleukin. Increased risk of hyperkalaemia with ciclosprin and tacrolimus. Increased risk of gouty arthritis with ciclosporin
Muscle relaxants - enhanced hypotensive effect with baclofen or tizanidine.
Increased effect of curare-like muscle relaxants
Oestrogens - diuretic effect antagonised
Progestogens (drosperidone) - increased risk of hyperkalaemia
Prostaglandins - enhanced hypotensive effect with alprostadil
Sympathomimetics - increased risk of hypokalaemia with high doses of beta2 sympathomimetics
Theophylline - enhanced hypotensive effect
Probenecid — effects of furosemide may be reduced by probenecid and furosemide may reduce renal clearance of probenecid.
Anaesthetic agents - general anaesthetic agents may enhance the hypotensive effects of furosemide. The effects of curare may be enhanced by furosemide.
Alcohol - enhanced hypotensive effect
Laxative abuse - increases the risk of potassium loss
Others: Concomitant administration of aminoglutethimide may increase the risk of hyponatraemia.
4.6. Pregnancy and lactation
Pregnancy
There is clinical evidence of safety of the drug in the third trimester of human pregnancy & furosemide has been given after the first trimester of pregnancy for oedema, hypertension and toxaemia of pregnancy without causing fetal or newborn adverse effects. However, furosemide crosses the placental barrier and should not be given during pregnancy unless there are compelling medical reasons. It should only be used for the pathological causes of oedema which are not directly or indirectly linked to the pregnancy. The treatment with diuretics of oedema and hypertension caused by pregnancy is undesirable because placental perfusion can be reduced, so, if used, monitoring of fetal growth is required.
Lactation (see section 4.3)
Furosemide is contraindicated as it passes into breast milk and may inhibit lactation.
4.7. Effects on ability to drive and use machines
Reduced mental alertness, dizziness and blurred vision have been reported, particularly at the start of treatment, with dose changes and in combination with alcohol. Patients should be advised that if affected, they should not drive, operate machinery or take part in activities where these effects could put themselves or others at risk.
4.8. Undesirable effects
Undesirable effects can occur with the following frequencies: very common (> 1/10), common (> 1/100, < 1/10), uncommon (> 1/1,000, < 1/100), rare (> 1/10,000, <
1,000) and very rare (< 1/10,000, including isolated reports).
Blood and lymphatic system disorders:
Uncommon:
• thrombocytopenia Rare:
• Eosinophilia
• Leukopenia
• Bone marrow depression (necessitates withdrawal of treatment). The haemopoietic status should be therefore be regularly monitored.
Very Rare:
• aplastic anaemia or haemolytic anaemia
• agranulocytosis
Nervous system disorders
Rare:
• paraesthesia
• hyperosmolar coma
Endocrine disorder
Glucose tolerance may decrease with furosemide. In patients with diabetes mellitus this may lead to a deterioration of metabolic control; latent diabetes mellitus may become manifest. Insulin requirements of diabetic patients may increase.
Uncommon: visual disturbance Ear and labyrinth disorders
Hearing disorders and tinnitus, although usually transitory, may occur in rare cases, particularly in patients with renal failure, hypoproteinaemia (e.g. in nephritic syndrome) and/or when intravenons furosemide has been given too rapidly.
Cardiac disorders
Uncommon: Cardiac arrhythmias
Furosemide may cause a reduction in blood pressure which, if pronounced may cause signs and symptoms such as impairment of concentration and reactions, light headedness, sensations of pressure in the head, headache, dizziness, drowsiness, weakness, disorders of vision, dry mouth, orthostatic intolerance.
Hepatobiliary disorders
In isolated cases, intrahepatic cholestasis, an increase in liver transaminases or acute pancreatitis may develop.
Hepatic encephalopathy in patients with hepatocellular insufficiency may occur (see Section 4.3).
Vascular Disorder:
Rare:
• vasculitis
Skin and subcutaneous tissue disorders
Uncommon:
• Photosensitivity Rare:
Skin and mucous membrane reactions may occasionally occur, e.g. itching, urticaria, other rashes or bullous lesions, fever, hypersensitivity to light, exsudative erythema multiforme (Lyell’s syndrome and Stevens-Johnson syndrome), bullous exanthema, exfoliative dermatitis, purpura, AGEP (acute generalized exanthematous pustulosis) and DRESS (Drug rash with eosinophilia and systemic symptoms)..
As with other diuretics, electrolytes and water balance may be disturbed as a result of diuresis after prolonged therapy. Furosemide leads to increased excretion of sodium and chloride and consequently increase excretion of water. In addition, excretion of other electrolytes (in particular potassium, calcium and magnesium) is increased. Metabolic acidosis can also occur. The risk of this abnormality increases at higher dosages and is influenced by the underlying disorder (e.g. cirrhosis of the liver, heart failure), concomitant medication (see section 4.5) and diet.
Symptomatic electrolyte disturbances and metabolic alkalosis may develop in the form of a gradually increasing electrolyte deficit or e.g. where higher furosemide doses are administered to patients with normal renal function, acute severe electrolyte losses,
Symptoms of electrolyte imbalance depend on the type of disturbance:
Sodium deficiency can occur; this can manifest itself in the form of confusion, muscle cramps, muscle weakness, loss of appetite, dizziness, drowsiness and vomiting.
Potassium deficiency manifests itself in neuromuscular symptoms (muscular weakness, paralysis), intestinal symptoms (vomiting, constipation, meterorism), renal symptoms (polyuria) or cardiac symptoms. Severe potassium depletion can result in paralytic ileus or confusion, which can result in coma.
Magnesium and calcium deficiency result very rarely in tetany and heart rhythm disturbances.
Serum calcium levels may be reduced; in very rare cases tetany has been observed. Nephrocalcinosis/Nephrolithiasis has been reported in premature infants.
Serum cholesterol (reduction of serum HDL-cholesterol, elevation of serum LDL-cholesterol) and triglyceride levels may rise during furosemide treatment. During long term therapy they will usually return to normal within six months,
As with other diuretics, treatment with furosemide may lead to transitory increase in blood creatinine and urea levels. Serum levels of uric acid may increase and attacks of gout may occur.
The diuretic action of furosemide may lead to or contribute to hypovolaemia and dehydration, especially in elderly patients. Severe fluid depletion may lead to haemoconcentration with a tendency for thromboses to develop.
General disorders and administration site conditions
Uncommon: Fatigue
Rare:
• Severe anaphylactic or anaphylactoid reactions (e.g. with shock) occur rarely.
• fever
• Malaise
Gastrointestinal disorders
Uncommon: dry mouth, thirst, nausea, bowel motility disturbances, vomiting, diarrhea, constipation.
Gastro-intestinal disorders such as nausea, malaise or gastric upset (vomiting or diarrhoea) and constipation may occur but not usually severe enough to necessitate withdrawal of treatment.
Rare:
Acute Pancreatitis
Renal and urinary disorders
Uncommon:
• serum creatinine and urea levels can be temporarily elevated during treatment with furosemide.
Rare:
• interstitial nephritis, acute renal failure.
Increased urine production, urinary incontinence, can be caused or symptoms can be exacerbated in patients with urinary tract obstruction. Acute urine retention, possibly accompanied by complications, can occur for example in patients with bladder disorders, prostatic hyperplasia or narrowing of the urethra.
Pregnancy, puerperium and perinatal conditions
In premature infants with respiratory distress syndrome, administration of Furosemide Accord Tablets in the initial weeks after birth entails an increased risk of a persistent patent ductus arteriosus.
In premature infants, furosemide can be precipitated as nephrocalcinosis/kidney stones.
Rare complications may include minor psychiatric disturbances.
4.9. Overdose
Features
Overdose can cause massive diuresis resulting in dehydration, volume depletion and electrolyte disturbances with consequent hypotension and cardiac toxicity. The clinical picture in acute or chronic overdose depends primarily on the extent and consequences of electrolyte and fluid loss, e.g. hypovolaemia, dehydration, haemoconcentration, cardiac arrhythmias due to excessive diuresis. Symptoms of
these disturbances include severe hypotension (progressing to shock), acute renal failure, thrombosis, delirious states, flaccid paralysis, apathy and confusion.High doses have the potential to cause transient deafness and may precipitate gout (disturbed uric acid secretion).
Management
• Benefits of gastric decontamination are uncertain. In patients presenting within 1 hour of ingestion, consider activated charcoal (50g for adults: 1g/kg for children)
• Observe for a minimum of 4 hours - monitor pulse and blood pressure.
• Treat hypotension and dehydration with appropriate IV fluids
• Monitor urinary output and serum electrolytes (including chloride and bicarbonate). Correct electrolyte imbalances. Monitor 12 lead ECG in patients with significant electrolyte disturbances
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: High-ceiling diuretic sulfonamides, loop diuretics;
ATC code: C03CA01
The evidence from many experimental studies suggests that furosemide acts along the entire nephron with the exception of the distal exchange site. The main effect is on the ascending limb of the loop of Henley with a complex effect on renal circulation. Blood-flow is diverted from the juxta-medullary region to the outer cortex.
The principle renal action of furosemide is to inhibit active chloride transport in the thick ascending limb. Re-absorption of sodium, chloride from the nephron is reduced hypotonic or isotonic urine produced.
It has been established that prostaglandin (PG) biosynthesis the renin-angiotensin system are affected by furosemide administration and that furosemide alters the renal permeability of the glomerulus to serum proteins.
5.2. Pharmacokinetic properties
Approximately 65% of the dose is absorbed after oral administration. The plasma half-life is biphasic with a terminal elimination phase of about 1/ hours. Furosemide is up to 99% bound to plasma proteins and is mainly excreted in the urine, largely unchanged, but also excreted in the bile, non-renal elimination being considerably increased in renal failure. Furosemide crosses the placental barrier and is excreted in the milk.
Furosemide is a weak carboxylic acid which exists mainly in the dissociated form in the gastrointestinal tract. Furosemide is rapidly but incompletely absorbed (60-70%) on oral administration and its effect is largely over within 4 hours. The optimal absorption site is the upper duodenum at pH 5.0. Regardless of route of administration 69-97% of activity from a radio-labelled dose is excreted in the first 4 hours after the drug is given. Furosemide is bound to plasma albumin and little biotransformation takes place. Furosemide is mainly eliminated via the kidneys (80-90%); a small fraction of the dose undergoes biliary elimination and 10-15% of the activity can be recovered from the faeces.
In renal/ hepatic impairment
Where liver disease is present, biliary elimination is reduced up to 50%. Renal impairment has little effect on the elimination rate of furosemide, but less than 20% residual renal function increases the elimination time.
The elderly
The elimination of furosemide is delayed in the elderly where a certain degree of renal impairment is present.
New born
A sustained diuretic effect is seen in the newborn, possibly due to immature tubular function.
5.3 Preclinical safety data
No further information available.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Lactose monohydrate Povidone K-30 Magnesium Stearate Sodium Starch Glycollate (Type A)
Maize Starch
6.2 Incompatibilities
Not applicable
6.3 Shelf life
Polypropylene containers/glass bottles: 3 years Blister strips: 2 years
6.4 Special precautions for storage
Container pack: Do not store above 25°C. Keep the container tightly closed.
Keep the container in the outer carton.
Bottle pack: Do not store above 25°C. Keep the bottle tightly closed. Keep the bottle in the outer carton.
Blister pack: Do not store above 25°C. Store in the original package in order to protect from light
6.5 Nature and contents of container
Polypropylene containers, with snap-on polythene lids, with integral tear-off security lids OR Glass bottles with screw caps with sternan faced liner: 1000,500,250 , 100, 84,70,54,42,28,21,15 and 14 tablets.
Blister strips (strips composed of aluminium foil and PVdC coated PVC film): 14, 15,21,28,42,56, 70 and 84 tablets.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
None stated
7 MARKETING AUTHORISATION HOLDER
Accord Healthcare Limited Sage House, 319 Pinner Road North Harrow, Middlesex HA1 4HF United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 20075/0052
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
24/02/2009
10 DATE OF REVISION OF THE TEXT
17/09/2014