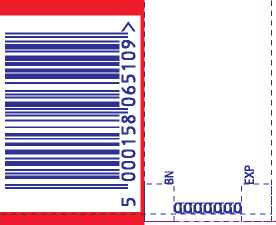
Gaviscon Advance Peppermint Flavour Oral Suspension
Reckitt Benckiser
What is this product? Each 10 ml contains sodium alginate 1000 mg and potassium hydrogen carbonate 200 mg.
Dosage: Check that the cap seal is unbroken before first using this product. Shake well before use. Read the package leaflet before use. For oral use. Adults, including the elderly and children 12 years and over: Take 5-10 ml (one to two 5 ml spoonfuls) after meals and at bedtime. Children under 12 years: Should only betaken on medical advice. If symptoms persist after 7 days consult your doctor. Contains sodium, potassium, calcium, methyl (E218) and propyl (E216) para-hydroxybenzoates. See leaflet for further information.
Do not refrigerate.
Keep out of the reach and sight of children.
Do not use this product after the expiry date (EXP: month/year shown). Use within 6 months of opening.
Peel here. Do not remove.
e 500ml


Manufacturer and Product Licence holder in the UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
UK Distributor: Forum Health Products PL00063/0612
GAVISCON
ADVANCE
•Hbsm?
sodium alginate potassium hydrogen carbonate
Heartburn & Indigestion • Extra Strength Formula •


GairisconAdrancePeppennitHeMairOialSuspensiaiL
conditions such as hiatus hernia (protrusion ofmusclethnoughamusclewalll.reflux
Read al elite leaflet cairiully because it certains mpcifertiiiniialinn for yoii
This medicine is available without prescription However, you si need to lake ibis medicine carefulyto getthe best results frail it
throat and cough associated with reflux, ftcanbetakento control heartfxim symptoms wfiichmayoccurwfientaking.or following withdrawal of medicationtD reduce stomach acid such as Proton Pump Inhibitor (PPIs| or H2
Askyour pharmacist ifyDuneedmareinfbrmab'onor advice.
You inrtcoiitactadoctwilyoirsingliimsworsen orrto notknproveafter7days.
1. What is Gaviscon Advance and what is it used lor? I Befbretaking this medicine.
3. Howtotakethismedicine 14. Possible side effects.
5. HowtD stDre tiis medicine.
6. Ftirtherinformatian.
2 Betetalam this medicine Do nottake Gaviscon Advance
- If vdu knowytxi are allergic (hypersensitive) to any of the ingredierits in this product (see Turther Information'forafull list of iigredientsl.
TakB special cats with Ganriscan Advance:
This medicine contaitssodium(4.6 mmol per 10 ml). patassiim (20mmol per 10ml) and calcium.
- If you have been advised to follows diet restricted in arryofthese please consultyour doctor before taking this product
-Please alsotakto your doctor regarding these salt contents if you suffer or have suffered from
; This product tones a protective layer thatfloals on top of the stomach contents. This layer prevents reflux and keeps the stomach contents awayfrom the lining of the food pipe to relieve
airy other medicines, including medicines obtained without prescription, hepancyandbreastfeerif You can take this product if you are pregnantor breast-feeding. As with all medicines, the
RB48720


Driving and using machaies
This medicine has no influence on the abiity to drive and use machres. 1 How In take this mafciie
ilmporiafadonnaliati abut same oftmihspSenfeSfoviscon Advance:
1 This product contains methyl (E218I and propyl (E216) para-hydroxybenzoates which may cause
Do not use this product after the expiry dade (EXP: montlVyear) shown. Use within 6 months of opening. Keep out of the reach and sight of children. Do not refngerale.
AdrtfcinchBhgflieelderiy and chihhen12yeart ail over 5-IOml(oneto two 5ml spoonfuls) after meals and at bedtime.
Children under I2years Should only be taken on medical advice
If you take too much of this product youmay feel bloated. It is unlkeiyto cause you any harm,but please consultyour doctor or pharmacist if this doesn't go away. flsymytomspeisistalter7dayscansrttyoiirdocto[
ingredients. Symptoms of this may include skin rash,itching, difficulty breathing, dizziness or swellrg of the face,lips,tongue orthroatlyouesrperiencefliese or any other side effect stop takmgthe product and consukyom doctor mmediately.
Scheme at 'httpy/irvvvw:mhragov.ul^ellovvcarir. By reporting side effects, you can help provide more|nformation on the salelypfths mmlicre.___
envTtmment 6. Fuller Irtoimrtiim
Each 10 ml of oral suspension contaiis 1000 mg sodium algiiate and 2tt) mg potassium hydrogen cartMnate as the active ingredients The other ingredients are calcium carbonate, carbotner, methyl (E218) and prapyi (E216) parahydroxybenzoates, sodium saccharin, sodium hydroxide, Peppermintflavour and purified water.This product does not contaki sugar or gluten.
Gaviscon Advance is available in bottles of 150 ml, 250 ml, 300 ml or 500 ml only from the
Manufacturer and Product Licence Holden Reckitt Benckiser Healthcare (UK) limited, Hull HU8 7DS.
Export Distributors: Reckitt&Coknan (Overseas) Limited, Hull HU870S. Date of preparation: May 2014
Gaviscon Advance and the sword and circle are trademarks.
PRINTING READS This Way

RB48720
What is this product? Each 10 ml contains sodium alginate inoo mg and notassium hydrogen carky'mte 200 mg.
Dosage: Check‘hit the crp seal is lmbro' en before first using this product. Shake well before use. Reat t' a Itjf Jt jefoi j use. Tor ol jI -s
Adults, incLdii.g thb elder!, a,.6 jhildrei. 12yet..s and over: Take J-10 .nl (oneto iwo o ml spool, fuls) after meals and at bedtime. Children under 12 yearn: should only he taken on memcsl advice. If symptom*' persist a^e- 7 days nr ns- lit your doctor. Contains sodium, potassium, calcium, methyl (E21H anr' pro; yl (E?16) para-h\ 1rr xyben 3c See letle,Lrfurtht: informat’on.
Do not refrigerate.
Keep out of the reach and sight of children.
Do not use this product after the expiry date (EXP: month/year shown). Use within 6 months of opening.
sodium alginate potassium hydrogen carbonate Heartburn & Indigestion Extra Strength Formula
annnnnn
GawsconAdvBncePeppeiinMFIavDurOiBlSuspengim.
Sodium alginate and potassium hydrogen carbonate.
Readall aftisIcaAetcaiGfiillybecaueitcanlainsimpaitantiniannalionlaryDU.
This medicine is available without presc' ' carefully to get the best results from it
conditions such as hiatus hernia (protrusion of muscle through a muscle wall), reflux
ttimertandcough associated with rofliix.
It can betaken to control heartburn symptoms which may occurvtrhen taking, orfotoving
Myourphannacist if you need more information or advice. VinimustconlactadoctiirifyoursyiqplpitBworsenorilonatiifniveater7ilays.
If any of the side-effects gets serious, or if you notice any side-effect not listBd in this leaflet.
1. Whatis Gaviscon Advance and what is it used for? I Beforetakiigthismedicine.
3. Howtotakethismedicine.
4. Possible side effects.
5. Howtostorethismedicine.
6. Further iiformalion.
1. What is this mericire and what is it used far?
'Further Infbrmatiort'for a full list of ingredients).
Tata special care wifo Gaviscon Advance:
Ibis medicine contains sodium 14.6 mmol perlO ml), potassium (2X1 mmol per 10 ml) and calcium. -If you have been advised to tbikwadiet restricted it any of these please consutyour doctor beforetaking 1his product
This pmductfbmis a protective layerthatfloatsontep of the stomach contents. This layer prevents reflux and keepsthe stomach contents away tom the lining of the food pipeto relieve
any other medicines, including medicines obtained without prescription.
Pregnancy and breast-feed iig You can take this product if youarepregnant or breast-feeding. As with all medicines,the treatmentduration should be limited as much as possible.
RB48720


Driving and usiq machines:
Thismedicinehasno influence on the ability to drive and use machines. IHowtotakeffrisniericine
I symptoms persist aftar7days const* yoridoctor. 4Pnssftle side effects
IhtjXKtannriloimalibnahautsoireartherrigredierBdGaviscon Advance
| This product contains methyl (E218) and propyl (E216) para-hydroxybenzoates which may cause
Alhdtnadiidngthe elderly and children 12yeaisand over 5-IOml (one to two 5ml spoonfuls) after meals and at bedtime.
Children under 12years: Should only be laken on medical advice.
ff you forgetadoseitis not necessary to double the dose nexttm,just cany on taking as before.
If you ta ke too much of this product you may feel bloated. It is trlikelytD cause you any harm,but
ingredients Symptoms ofthis may include skit rash, itching, difficulty breathing, dizziness or sweling of the face,lips, tongue orthroat II you experience these or any other side effect stop takingtopraduct and consrdtyow doctor miwiataly.
If you get any side effects, takto your doctor, pharmacist or nurse. This includes any posable
Scheme at Tttjp/Mwwmhra,gov.u^ellowcard'. By reporting side effects, you can help provide more rfomiation on ttte ^fet^pfthte medicine.___
Do not use this product aftertbe expiry date (EXFh montfVyear) shmvn. Use within B months of opening Keep out of the reach and sight of children Do notnririgerala
environment 6. Father Hornietion
carbonate asthe active ingredients. The other ingredients are calcium carbonate, carbomer, methyl (E218I and propyl (E216I parahydroxybenzoates, sodium saccharin, sodium hydroxide, Peppermintflavour and purified water. This product does not contain sugar or gluten. Gaviscon Advance is available in bottles of 150 ml,250 mf 300 ml or 500 ml only from the pharmacy. Not all pack sizes may be marketed.
Manufacturer and Product licence Holder Reckitt Benckiser Healthcare (UK) limited, Hull, HU87DS.
Export Distributors: Reckitt & Cdman (Overseas) Limited, Hull HU8 7DS.
Gaviscon Advance and the sword and ciele aretrademarks.
PRINTING READS This Way

RB48720

What is this product? Each 10 ml contains sodium alginate 1000 mg and potassium hydrogen carbonate 200 mg.
Dosage: Check that the cap seal is unbroken before first using this product. Shake well before use.
Read the package leaflet before use.
For oral use. Adults, including the elderly and children 12 years and over: Take 5-10 ml (one to two 5 ml spoonfuls) after meals and at bedtime. Children under 12 years: Should only be taken on medical advice. If symptoms persist after 7 days consult your doctor. Contains sodium, potassium, calcium, methyl (E218) and propyl (E216) para-hydroxybenzoates. See leaflet for further information.
Do not refrigerate.
Keep out of the reach and sight of children. Do not use this product after the expiry date (EXP: month/year shown). Use within 6 months of opening.
Peel here. Do not remove.


Manufacturer and Product Licence holder in the UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
ADVANCE

sodium alginate potassium hydrogen carbonate Heartburn & Indigestion Extra Strength Formula
jCaviscon Advance Peppenni i RavotiDral Suspension.
M all oftois leaflet carehdly because it contains important infamatioiifof you,
this medicine is available without prescription. However,
K Riithennfbimation.
t. What is this medicine and wh* is it iKcHfw?
Gaviscon Advance belongs to a group of medicines called
results from it v
• Askyour pharmacistifyou need more information or \ advice.
■ You must contactadodor if yon symptoms worsen or ! do not improve alter 7 days.
' If any of tne side-effects gets serious, or if you notice
i any side-effect not listed inthis leaflet pleasetellyour ! doctor or pharmacist fa this leaflet
X What is Gaviscon Advance and what is it used for?
2. Before takingthis medicine.
X Howto take this medicine.
4. Possible side effects, o. Howto store this medicine.
heartburn symptoms which may occurwhile taking, or following withdrawal of medication to reduce stomach acid such as Proton Pump Inhibitors (PPI's) or H2Antagonists.
2 Befforelaldnqthis meiBcine Do nottake Gaviscon Advance:
- If you know you are allergic (hypersensitive)to any of the ingredients in this produrtisee further Information' for a fuIliflofmg_redients)._ _
13ie special care wiffiGaviscon Advance:
This medicine contains sodium (4.6 mmol per 10 ml), potassium (20 mmol per 10 ml) and calcium.
- If you have been advised to follow a diet restricted in any of these please consultyour doctor before taking this product
- Please also talkto your doctor regarding these salt contentsifyousuflerorhavesuffered from significant kidney or heart disease, as certain salts could interfere with these diseases.
Taking other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any cither medicines, including medicines obtained without prescription.
Pregnancy and breastfeeding:
You can take this product ifyou are pregnant or breast-feeding. As with all medicines, the treatment duration should be limited as much as possible.
-RB48732
" inportantlnfemtalian about some offfte ingredems of ~ Gniscon Advance:
This product contains methyl (E2T8) and propyl (E216)
Drivhg and using machines
This medicine has no influence on the abifity to drive and
1 Howtatakethismedicine
Checkthattfie cap seal is unbn product Shake well before use. Adults including the" elderly and children 12 years and over 5-IOml (one to two
Children under 12years: Should only be taken on medical advice. If you forget a dose it is not necessary to double
take too much ofthis product you mayfeel bloated. It is unlikely to cause you any harm, but please consultyour
ffsymptoms persist afterTdays consult yon doctor
4iTh>ssiile side effects

you experience these or any othershle effects slop taking the product and consult yoiidoctcr immediately.
inthisleafletYoucanal the YellowCard Scheme at ■httpY/www.mhra.gov.uk/yellowcarcT. By reporting side effects, you can help provide more information on the
& Howto store this medicine
Do not use this product after the expiry date (EXP: month/year) shown. Use within 6 months of opening. Keep outofthe reach and sight of children.
Donotrefrigerale.
medicines no longer requMlhese measures will help ' to protectthe environment
6. Fiitherkrformation 1
Each 10 ml of oral suspension contains 1000 mg sodium ! alginate and 200 mg potassium hydrogen carbonate as \ the active ingredients. The other ingredients are calcium i carbonate, carbomer, methyl (E218) and propyl IE216) \
parahydroxybenzoates,sodiumsaccharin,sodium i hydroodde, Peppermintflavourand purified water. This \ product does not contain sugar or gluten. (
Manufacturer and Product Licence Holder \
Reckitt Benckiser Healthcare (UK) L'mited, Hull, HU87DS.' Export DistributorsrReckrtt&Calman (Overseas) Limited, \ Hull, HUS7DS.
Darts of preparation: May 2014. !
Garviscon Advance and the sword andcircleare \ trademarks. i
____________________RB48732!
PRINTING READS This Way
RB48732
What is this product? Each 10ml contains sodium alginate 1000 mg and potassium hydro^e^ c^bon^te 200 mg.
Dosare: Checkth°t the ~ap spal is unbroken before first using thr product Shake wel1 before use.
Read p package hefce usp
For or»l use. Adul% incuamg the p'd°rly and children 12 years ^nd O',er:Take5-'l0 ml 'one to two 5 ml spoonfuls) after meals ana at bedtime. Children under 1?year.c- Sho"ld on'y be faken on medical advice. If symptoms oersist a«:er 7 days consuh your doctor. Contains sodium, potassium, calcium, methyl (1=218) and propyl (E2161 oara-hydroxybenzoates. See leaflet for further information.
Do not refrigerate.
Keep out of the reach and sight of children.
Do not use this product after the expiry date (EXP: month/year shown). Use within 6 months of opening.
Peel here. Do not remove.
@e300ml

Raviscon Advance Peppeimint RavwDral Suspension.
Read all oflhis leaflet carefully because it curtains foportantiidonnatiixifofyrxi,
iThis medicine is available without prescription. However,
K FuitherTnformalon.
1. What is this medicine and wtnesitiKgUi*?
Gaviscon Advance belongs to a group of medicines called
results from it
]> KeeptbisleafleLYoumayneedtoreaditagain. a Askyour pfiarmacistrfyau need more information or ! advice.
■ Vaumustcontactadodor ifyairsymptomsworsenor ! do not nnnive after 7 days.
' if any of trie side-effects gets serious, or if you notice
i any side-effect not listed in this leaflet, please tell your ' doctor nr pharmacist In this leaflet
[1. What is Gaviscon Advance and what is it used for?
2. Before taking this medicine.
S. Howto take this medicine.
A. Possible side effects o. Howto store this medicine.

heartburn symptoms which may occurwhile taking, or following withdrawal of medication to reduce stomach acid such as Proton Pump Inhibitors (PPfs) or H2Arrtagonists 2. Before taking this mericine Do not take Gaviscon Advance:
■ Ifyou know you are allergic (hypersensidve)toanyofthe ingredients in this productjsee further Information' for a
lake special care wHiSavisconTUvance:
This medicine contains sodium mmol per 10 ml), potassium (ZO mmol per 10 ml) and calcium.
- If you have been advised to follow a diet restricted in any of these please consultyour doctor before taking this product
- Please also talkto your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salts could interfere with these diseases
Takkig other medicines;
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines including
medicines obtained without prescription. Pregnancy and breast-feeding You can take this product ifyou are pregnant or
fullstoffogrodienfo).
RB4SZ32
fnportantTnformation about some offhe ingrecients of ~ Gaviscon Advance
This product contains methyl (E218) and propyl (E216)
yfosSffesiijeeffe* f:’". 1 Bin
of an allergic reaction to the ingredients. Symptoms of this may include skin rash, itching, difficulty breathing,
Driving and using machines
This medicine has no influence on the ability!) drive and use machines
1 Howto lake this medicine
you experience these or any otherside effects slop taking the product and consult yoridoctofinmerSately.
product Shake well before use. Adults including the etderly and children 12years and over 5-IOml (one to two
Children under 12yeaix Should only be taken on medical advice.......
or nurse. I inthisleaflet You the YellowCard Scheme at ■TTttpvywwwrnhra.gov.uk/yellowcanf. By reporting side effects you can help provide more information on the
Litis
ffsymptons persist afferf days consult you doctor
& Howto store this medicine
Do not use this product afterthe expiry date (EXP: month/year) shown. Use within 6 months ofopening.Keep out of the reach and sight of children.
Do not refrigerate.
medicines no longer required.TfTese measures will help ' to protect the environment
the active ingredients. The other ingredients are calcium i
carbonate, carbomer, methyl (E218) and propyl (E216) |
parahydroxybenzoates, sodium sacchar'asodium '
hydroxide, Peppermintflsrvourand purified water.This \
product does not contain sugar or gluten. j
Manufacturerand Product Licence Holden )
Reckitt Benckiser Healthcare (UK) Umited,Hull, HU87DS.' Export Distributors: Reckitt & Colman (Overseas) Umited, \ Hull, HU87DS.
Date of preparation: May 2014. !
Gaviscon Advance and the sword andcircleare (
____________________RB4S732I
PRINTING READS This Way
RB48732

What is this product? Each 10 ml contains sodium alginate 1000 mg and potassium hydrogen carbonate 200 mg.
Dosage: Check that the cap seal is unbroken before first using this product. Shake well before use.
Read the package leaflet before use.
For oral use. Adults, including the elderly and children 12 years and over: Take 5-10 ml (one to two 5 ml spoonfuls) after meals and at bedtime. Children under 12 years: Should only be taken on medical advice.
If symptoms persist after 7 days consult your doctor. Contains J sodium, potassium, calcium, methyl (E218) and propyl (E216) para-hydroxybenzoates.
See leaflet for further information.
“move, e 250ml
Do not refrigerate.
Keep out of the reach and sight of children.
Do not use this product after the expiry date (EXP: nionth/year shown]. Use within 6 months of opening. Manufacturer and Product Licence holder in the UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS.
UK Distributor:
Forum Health Products PL00063/0612
ADVANCE
'Hkmn?
sodium alginate potassium hydrogen carbonate Heartburn & Indigestion Extra Strength Formula
__m_______£L _i_
J _ _ smmm.. _ \
_l_I_I_L
\n/\ Gaviscon Advance Peppermint Flavour Oral suspension.
Sodium alginate and potassium hydrogen carbonate. Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to take this medicine carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve after 7 days.
If any of the side-effects gets serious, or if you notice any side-effect not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What is Gaviscon Advance and what is it used for?
2. Before taking this medicine.
3. Howto take this medicine.
4. Possible side effects.
5. Howto store this medicine.
6. Further information.
1. What is this medicine and what is it used for?
Gaviscon Advance belongs to a group of medicines called 'reflux suppressants'.
This product forms a protective layer that floats on top of the stomach contents. This layer prevents reflux and keeps the stomach contents away from the lining of the food pipe to relieve the symptoms of heartburn and acid indigestion. It can also be used to relieve the symptoms of conditions such as hiatus hernia
[protrusion of muscle through a muscle wall), reflux oesophagitis (inflamed foodpipe) and symptoms of hoarseness and other voice disorders, sore throat and cough associated with reflux.
It can be taken to control heartburn symptoms which may occur when taking, or following withdrawal of medication to reduce stomach acid such as Proton Pump Inhibitors (PPIs) or H2 Antagonists.
2. Before taking this medicine Do not take Gaviscon Advance:
- If you know you are allergic (hypersensitive) to any of the ingredients in this product (see 'Further Information' for a full list of ingredients).
Take special care with Gaviscon Advance:
This medicine contains sodium (4.6 mmol per 10 ml), potassium (2.0 mmol per 10 ml) and calcium.
- If you~have Been advisetfto follow a diet restrfctedln any of these please consult your doctor before taking this product.
-Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salts could interfere with these diseases.
Taking other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without prescription Pregnancy and breast-feeding:
You can take this product if you are pregnant or breast-feeding. As with all medicines, the treatment duration should be limited as much as possible.
portant information about some of the Ingredients of Gaviscon Advance:
This product contains methyl (E218) and propyl (E216) para-hydroxybenzoates which may cause allergic reactions (possibly delayed).
Driving and using machines:
This medicine has no influence on the ability to drive and use machines.
3. How to take this medicine
Check that the cap seal is unbroken before first using
this product. Shake well before use.
Adults including the elderly and children 12 years and over: 5-10 ml (one to two 5ml spoonfuls) after meals and at bedtime. Children under 12 years: Should only be taken on medical advice. If you forget a dose it is not necessary to double the dose next time, just .carry on takings before,, If you_take_tpo muchofthis _
product you mayfeefbloatecf.Itls unlikely to cause you any harm, but please consult your doctor or pharmacist if this doesn't go away.
If symptoms persist after 7 days consult your doctor.
4. Possible side effects
Very rarely (less than 1 in 10,000 patients) there is a chance of an allergic reaction to the ingredients. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness or swelling of the face, lips, tongue or throat. If you experience these or any other side effects stop taking the product and consult your doctor immediately.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at:
" h ttp y/yvww. m h r a. gov. u k/y e 11 o wc a r d \ By reporting _ _
side effects, you canTielp provide more Information on the safety of this medicine.
5. How to store this medicine
Do not use this product after the expiry date (EXP: month/year) shown. Use within 6 months of opening. Keep out of the reach and sight of children. Do not refrigerate.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist howto dispose of medicines no longer required. These measures will help to protect the environment.
6. Further Information
Each 10 ml of oral suspension contains 1000 mg sodium alginate and 200 mg potassium hydrogen carbonate as the active ingredients. The other ingredients are calcium carbonate, carbomer,
methyllEZIS) and propyllE216) parahydroxybenzoates, sodium saccharin, sodium hydroxide. Peppermint flavour and purified water. This product does not contain sugar or gluten.
Gaviscon Advance is available in bottles of 150 ml,
250 ml, 300 ml or 500 ml only from the pharmacy.
Not all pack sizes may be marketed.
Manufacturer and Product Licence Holder:
Reckitt Benckiser Healthcare (UK) Limited,
Hull, HU8 7DS.
Export Distributors: Reckitt & Colman (Overseas) Limited, Hull, HU8 7DS.
Date of preparation: May 2014.
Gaviscon Advance and the sword and circle are trademarks.

U
What is this product? Each 10 ml contains sodium alginate 1000 mg and potassium hydrogen carbonate 200 mg Dosage: Check th^t the cap seal is unbroken before first using this product. Shake well before use. Read tee pacKaqe >earlet oerore use. For ornl use. Aduhs, incuding the elderly and children 12 years and
Do not refrigerate.
Keep out of the reach and sight of children.
Do not use this product after the expiry date
(EXP: month/year shown), Use within 6 months of opening,
Manufacturer and Product Licence holder in the UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS.
UK Distributor:
Forum Health Products
PL00063/0612 I-
spoonruis) utter meals and at bedtime. Children undei 12 years: Should only oe taken on medical advice.
If symotums persist after '/ days consult your doctor. Contains sodium, poidSaium, caluum, methyl (E218) and propyl (E216) para-hydroxybenzoates.
See leaflet for further information.
sodium alginate potassium hydrogen caroonate Heartburn & Indigestion
Do not remove. e 250ml
Extra Strength Formula
______|ia__
j _ _ jBHffiSjZSZIZ _ _ J
\m?\ Gaviscon Advance Peppermint Flavour Oral ^ suspension.
Sodium alginate and potassium hydrogen carbonate. Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to take this medicine carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve after 7 days.
If any of the side-effects gets serious, or if you notice any side-effect not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What is Gaviscon Advance and what is it used for?
2. Before taking this medicine.
3. How to take this medicine.
4. Possible side effects.
5. How to store this medicine.
6. Further information.
1. What is this medicine and what is it used for?
Gaviscon Advance belongs to a group of medicines called 'reflux suppressants'.
This product forms a protective layer that floats on top of the stomach contents. This layer prevents reflux and keeps the stomach contents away from the lining of the food pipe to relieve the symptoms of heartburn and acid indigestion. It can also be used to relieve the symptoms of conditions such as hiatus hernia
[protrusion of muscTe through a muscle wall), reflux oesophagitis (inflamed foodpipe) and symptoms of hoarseness and other voice disorders, sore throat and cough associated with reflux.
It can be taken to control heartburn symptoms which may occur when taking, or following withdrawal of medication to reduce stomach acid such as Proton Pump Inhibitors (PPIs) or H2 Antagonists.
2. Before taking this medicine Do not take Gaviscon Advance:
- If you know you are allergic (hypersensitive) to any of the ingredients in this product (see 'Further Information' for a full list of ingredients).
Take special care with Gaviscon Advance:
This medicine contains sodium (4.6 mmol per 10 ml), potassium (2.0 mmol per 10 ml) and calcium.
- If youhave been advisecfto follow a diet restrictedln any of these please consult your doctor before taking this product.
-Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salts could interfere with these diseases.
Taking other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without prescription Pregnancy and breast-feeding:
You can take this product if you are pregnant or breast-feeding. As with all medicines, the treatment duration should be limited as much as possible.
Important luloruiation aTiout some of the Ingredients of Gaviscon Advance:
This product contains methyl (E218) and propyl (E216) para-hydroxybenzoates which may cause allergic reactions (possibly delayed).
Driving and using machines:
This medicine has no influence on the ability to drive and use machines.
3. How to take this medicine
Check that the cap seal is unbroken before first using
this product. Shake well before use.
Adults including the elderly and children 12 years and over: 5-10 ml (one to two 5ml spoonfuls) after meals and at bedtime. Children under 12years: Should only be taken on medical advice. If you forget a dose it is not necessary to double the dose next time, just carry on taking as before^ If you take too much of this
product you may feel Bloated". Ttls unlikely to cause you any harm, but please consult your doctor or pharmacist if this doesn't go away.
If symptoms persist after 7 days consult your doctor.
4. Possible side effects
Very rarely (less than 1 in 10,000 patients) there is a chance of an allergic reaction to the ingredients. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness or swelling of the face, lips, tongue or throat. If you experieoce these or any other side effects stop takiog the product and consult your doctor immediately.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: l'httpi//vwyw. mh ra^gov. u k/yel I owe a rd'l By reporting___
side effects, you canlielp provide more information on the safety of this medicine.
5. How to store this medicine
Do not use this product after the expiry date (EXP: month/year) shown. Use within 6 months of opening. Keep out of the reach and sight of children. Do not refrigerate.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further loformation
Each 10 ml of oral suspension contains 1000 mg sodium alginate and 200 mg potassium hydrogen carbonate as the active ingredients. The other ingredients are calcium carbonate, carbomer.
methyllE218) and propyl[E216) parahydroxybenzoates, sodium saccharin, sodium hydroxide. Peppermint flavour and purified water. This product does not contain sugar or gluten.
Gaviscon Advance is available in bottles of 150 ml,
250 ml, 300 ml or 500 ml only from the pharmacy.
Not all pack sizes may be marketed.
Manufacturer and Product Licence Holder:
Reckitt Benckiser Healthcare (UK) Limited,
Hull, HU8 7DS.
Export Distributors: Reckitt & Colman (Overseas) Limited, Hull, HU87DS.
Date of preparation: May 2014.
Gaviscon Advance and the sword and circle are trademarks.
|
• • |
• |
• |
• |
• |
• • |
• |
• • |
• |
• • |
• • |
• • | ||
|
• • |
• |
• |
• |
• |
• |
• |
• |
• | |||||
|
• • |
• |
• |
• |
• • |
• | ||||||||
|
• | |||||||||||||
|
• |
• |
• |
• |
• • |
• |
• |
• • | ||||||
|
• |
• |
• |
• |
• |
• | ||||||||
|
• • |
• |
• |
• |
• |
• |
• |
• |
• |
• | ||||
|
• |
• |
• |
• • |
• |
• • |
• | |||||||
|
• |
• • |
• |
• • |
• |
• |
• |
• | ||||||
|
• |
• |
• |
• |
• |
• |
• |
• | ||||||
|
• |
• • |
• |
• |
• |
• |
• |
1.3.2 Mock-up
Gaviscon Advance National Peppermint Flavour 160950
Page 6