Gaviscon Strawberry Flavour Tablets
usjn|B jo jeBns
uibjuoo jou op sjaiqe) asaqi jnoAep lueeja AjjaqMBJis ' (ZZ.13) apixo uoj; paj 'uimpos asoiiauues pue |0)i|Ax 'ajBJBajs lunjssuBeuj '(IS63)slub1jb(1sb 'OOO'OZ IoBojobiu '(lZfr3) |0)!uueui ajB siuajpajBuj jeqio aqi 'SiueipajBu! 3AIJ0B OipSB SJBUOqjBO LUn|3|33 BlUOg pUB 0JBUOqjB3 uaBojjiAq^iumpos Bu^^i '3}bu|B|b rnnipqs Bujqsj su|3]U03]3|qe)3|qeMaq3 qaeg AjjaqMBjjsjo jnoAeq pue jnopo aqi qpAA saBpa pa||0Aaq qj|M jsp 'jBinojp ^ujd 3|6d 3jb sj3|qej asaqi WOllVHHOdNI HHLUfid 'aujsjpaiu sjqijo Aisles eqi uo uopBiujo^ui ejoui apiAOjd d|eq ues noA 'siaaqa apjs Bupjodej Ag 'npjB3MO||aA/)|n'AoB'BjqLLi'A/\AAM//:djjqn
qe awaqps pJE^MOjia^ aqi B|A Apasjjp speqe ap;s yodaj os|B ubs nox tapeai s;Lp. u; pa]S|| jou siaaqa apis a|q;ssod Aub sapn|3U| siqi -asjnu jo ispBWjeqd tioioop jnoA 04. >||Bi 'sjoaya apis Aub jaB noA j| -A|aqe;paLuiu; jojoop jnoApnsuoa puB isnpojd aip Buo|ei dois sjaapa-apis jaqio Aub jo asaqi aouauadxa noAp qaojqijo anBuoj'sdji'asBi aqyo BuipaMS jo |ssauiRjp 'Bmqieajq Apnoipip 'Buiqop 'qsBj u|>|S apnpui Abuj siqi 10 siuoidujAs siua;pajBu| aqi 01 uopoBaj a;6ja||B ub 40 aaueqs (OOO'OL uj l ueqi ss3|) ajej Aja/\ ispapa apjs aiqjssog joiaop jnoA pnsuoo sAbp l Jape isisjad siuojdiuAs p qanpoid s;qi bu»|Bi japv qspewjeqd jo joiaop jnoApnsuoa asea|d inq 'ujjBq Aub noA esnea o; A|a>|||un s| p peieoiq |aaq Aew nqxqqnpqid siqyq qanuj po; a>|Bi noA y ajopaq sb
Bu|>|bi uo Ajjbo isnt 'aiup ixau asop aqi a|qnop ioi( oq :asop BiaBjojnoAp -aajApB |B3;paui uo us)|bj aq A|uo pinoqs isjBaA^i Japun uajppqg aiwiparj IB puB s|B3lu japB sia|qBi jnoj oi o/vq 0>|bi jnooo siuoidujAs uaqM jbao puB sjubAzi uajppqo pus A|jap|a aqi Bwpniauj ‘spnpv Buimo||bms sjo^aq; A|qBnqjqqi/y\aqg HjqqBJisiuiujqEjBjqjq-piafiBsofll
■s ssio/raoooid
s sai8nH'nnH 'm(>nl
= jnnpueg ipoau :)|fi
5-__ui japjpujidjpuB jnpjjjnuBjN
|
1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 | |||||||||||
|
PatientTnTomatibn Leaflet Gaviscon Strawberry Flavour Tablets Sodium alginate. Sodium hydrogen cnrbonate, Cnlcium carbonate [These tablets bring relief from the pain and discomfort of heartburn and acid indigestion, which for example, can [occur after meals or during pregnancy. This product 'belongs to a group of medicines called reflux |
suppressants, which form a protective Tayer on top of the stomach contents to prevent stomach acid escaping from the stomach where it works into the food pipe causing pain and discomfort. Before takinu this nroduct Do nut take if vou know that you are allergic to any of the ingredients (see further information for a full list). Each four tablet dose contains |
" TDTB mmoTof sodium and 3.2 mmol ofcalcium.lfyou have been advised to follow a diet restricted in either of these salts please consult your doctor before taking this product Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salts could interfere with these diseases. If you have |
phenylketonurTa, note that this product Ts sweetened-with aspartame, a source of phenylalanine. You can take this product if you are pregnant or breast-feeding. However, the treatment duration should be limited as much as possible. Taking other medicines: Do not take this product within two hours of taking other medicines by mouth as it can |
- interfere with the action of some other medicinesTThis Ts especially important if you are taking antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta blockers (for heart conditions), penicillamine (commonly for rheumatoid arthritis), glucocorticoid (for inflammatory and autoimmune disorders), neuroleptics (for mental illness), thyroid hormones, chloroquine (for malaria), estramustine (for prostate cancer) and bisphosphonates (for osteoporosis). | |||||||
|
i l l l l i i i i i i l l l i | |||||||||||
ujixisE |dqe~l aseg
Aexxsiqj,
SQVHH
DMUMlPd
Height
26mm
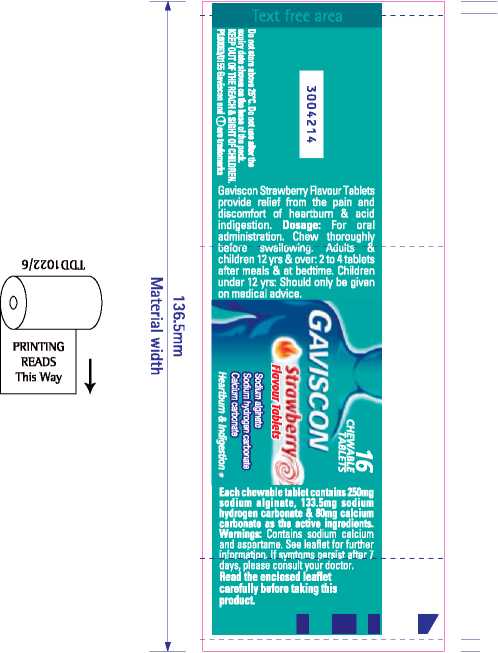
uajn|B jo jeBns umjuoa jou op siaiqei asaqi jnoAey uieajo AjjaqMGjjs' [ZLlj) ep|xo uoji paj 'uumpos aso||aujjeo pue |Ojj|Ax'eiejeais iun;sauBeui '(LS63)8Lue}jGdsG '000'02 |oBojobuj'(|0)iuueiu sjb spjajpajBuj jaqjo ai)l 'sjuaipajEu) eApae aqi se aieuoqjeo wma|eo Biuos pue ajeuoqjea ueBojpAq rnnipos Biugggi 'ajeuiB|e wmpos Bujqs£ sujejuqa }a|qe) aiqeMaqa yaej 'AjjagMej]s jp jnoAey
pue jnopo ayi qjiM saBpa pa||aAaq qpM je|j. 'jB|naj|a *HU)d a|Bd aje sjaiqej asaqi WOllVWHOdNI UBHUKU auioipaujsiqijoAiaies aqi uo uopeiujojui bjouj apiAOJd d|aq ueo noA 'siaajqa ap;s Bupjodaj Ag „pjeaMO||aA/)|n AoB ejqLU /wvw\//:dpqn qe aiuaqos pjeg mo||si aqj bia Apoajip sjoaya apisjjodaj osieueanpi qayea[siy} ui pajsi) iqu sjaaya
apis aiqjssod Aue sapn|au| siqi asjnu jo jspeujjeqd 'jojoop jnoA oq >||bj 'spaya apis Aue jaB noAy -A|aqe)paujuii jojoop jnoAqnsuoo pue jarpojd aqjBu^eidojs spaya-apjs jaqio Aue jo asaqi aouauadxa noA j| qeojyi jo anBuorsd)| 'aoe) a yip Buiusaas jo 'ssau|zz;p 'Bwqieajq Aynayyp 'Buiqoj; 'qsej up|s apnpu; Aeiu s|yijo siuoiduiAs ^siuajpajBui aqi oi uoi^ieaj ajBjsne uejo aaueya
(OOO'OLu! L ueqissai) ajej Aia/\ ispaya apjs a|q;ssog ■jopop jnoAnnsuoo sAep l Jaye isisjad siuoylLuAs II qanpojd sjip 6un|e| jayv qsiaeiweqd jo jopop jnoAi|nsuoo asea|d jnq 'uuey Aue noA asneo oi A|a>|i|un s) n 'paieoiq laaj. Aeuj noi qanpojd sjip jo qanui ooi a>|ei noA y ajojaq se 6ui>|eiuoAjjea isn[ 'amp jxau asop _ _ayi_aiqnqpiou og :esop Biafury noAjj oqiApBpoipaw
uo ua>|ei aq A|uo pinoqs :sjeaA zi Japun uajppqa ■aiuppaq ;e pue s|eaui jape siaiqej jnoqoi oaai aqej jnooo smoidmAs uaqy\/\ jsao pue sjeaAzi uejpi;qa{ pue A|jap|e aip Bujpn|ouj ‘synpv 'Buiaaoubaas ajojaq) AiqBnojoipAAaqg -uopejisjuiuipe |bjo joj :a6esog (sjsojodoaiso joj) saieuoydsoqdsjq pue (jaoueoi aiBjsojd joi| aui|snmBj]sa^BiJB|BUj jop aumbojq|qp|
■b
SSIO/E900C1J
■sga anH iriH "m Dim
ajB3ip|B3H J0S[^u0g _ uj jjpjpHjdjpuB MinjajjnuejN
|
1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 | |||||||||||
|
Patient riribnnaiionleailei Gaviscon Strawberrv Havour Tablets Sodium alginate. Sodium hydrogen caibonate. Calcium Carbonate These tablets bring relief from the pain and discomfort of jheartburn and acid indigestion, which for example, can occur after meals or during pregnancy. This product |
"belongs to a group ofmedicines caDedTeflux suppressants, which form a protective layer on top of the stomach contents to prevent stomach acid escaping from the stomach where it works into the food pipe causing pain and discomfort. Before takinn this nroduct Do not take if vou know that you are allergic to any of the ingredients (see further |
" information For a fuinistJT Each four tablet dose contains 10.6 mmol of sodium and 3.2 mmol of calcium. If you have been advised to follow a diet restricted in either of these salts please consult your doctor before taking this product. Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant |
"kidney orbeart disease, as certain salts could interfere with these diseases. If you have phenylketonuria, note that this product is sweetened with aspartame, a source of phenylalanine. You can take this product if you are pregnant or breast-feeding. However, the treatment duration should be limited as much as possible. |
"Taking other medicines: Do not take this product within two hours oftakmg other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta blockers (for heart conditions), penicillamine (commonly for rheumatoid arthritis), glucocorticoid (for inflammatory and autoimmune disorders), neuroleptics (for mental illness), thyroid hormones. | |||||||
|
i l l l l i i i i i i l l l i | |||||||||||
WUUS£
|dqe~l aseg

Height
26mm
00 cn o m —I > 33 33 < 33 > —
o
• •
• •
• • • •
• • • •
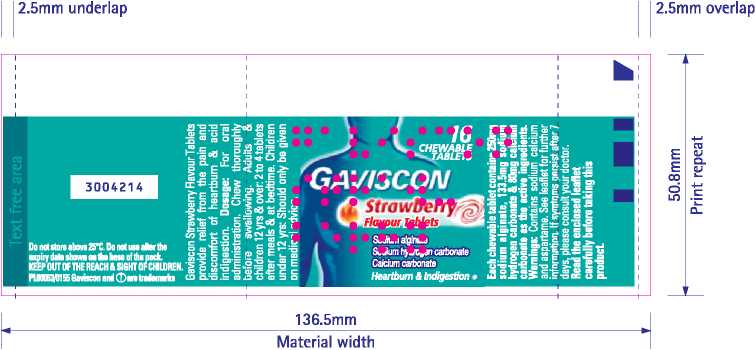
2.5mm underlap I I 2.5mm overlap
50.8mm Print repeat
• •
• •
• • •
• •• •
• •
• • • •
TBR1448-D8039779 - English
|
• • |
• |
• |
• |
• • |
• |
• • | ||
|
• • |
• |
• |
• |
• | ||||
|
• |
• |
• |
• | |||||
|
• |
• |
• |
• | |||||
|
• |
• • |
• |
• • | |||||
|
• |
• |
• |
• • | |||||
|
• |
• |
• |
• • |
• | ||||
|
• |
• • |
|
• • |
• |
• |
• |
• • |
• |
• • | ||
|
• • |
• |
• |
• |
• | ||||
|
• |
• |
• |
• | |||||
|
• |
• |
• |
• | |||||
|
• |
• • |
• |
• • | |||||
|
• |
• |
• |
• • | |||||
|
• |
• |
• |
• • |
• | ||||
|
• |
• • |
136.5mm Material width
TBR1448-D8039779 - English
|
• • |
• |
• |
• |
• • |
• |
• • | ||
|
• • |
• |
• |
• |
• | ||||
|
• |
• |
• |
• | |||||
|
• |
• |
• |
• | |||||
|
• |
• • |
• |
• • | |||||
|
• |
• |
• |
• • | |||||
|
• |
• |
• |
• • |
• | ||||
|
• |
• • |


1.3.1 Outer Packaging
Gaviscon Strawberry Tablets National 250mg

TBR1448-D8039779 - English
GAVISCON
STRAW
BERRY
Page 12