Gonapeptyl Depot 3.75mg Powder And Solvent For Suspension For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
GONAPEPTYL DEPOT 3.75mg, Powder and solvent for suspension for injection.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
One pre-filled syringe contains 3.75 mg triptorelin (as acetate) to be suspended in one ml sodium containing suspension agent.
The product contains 3.69 mg/ml equivalent to 0.160 mmol/ml sodium after reconstitution.
For a full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Powder and solvent for suspension for injection prolonged release in pre-filled syringes.
Visual description:
Before mixing: White to faintly yellow powder and a clear colour less aqueous liquid.
After mixing: Homogeneous milky white to faintly yellow suspension.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Men:
Treatment of hormone dependent locally advanced or metastatic prostate cancer.
Women:
Preoperative reduction of myoma size to reduce the symptoms of bleeding and pain in women with symptomatic uterine myomas.
Symptomatic endometriosis confirmed by laparoscopy when suppression of the ovarian hormonogenesis is indicated to the extent that surgical therapy is not primarily indicated.
Children:
Treatment of confirmed central precocious puberty (girls under 9 years, boys under 10 years).
4.2 Posology and method of administration
The product should only be used under the supervision of an appropriate specialist having requisite facilities for regular monitoring of response.
The treatment of children with triptorelin should be under the overall supervision of the paediatric endocrinologist or of a paediatrician or endocrinologist with expertise in the treatment of central precocious puberty.
It is important that the injection of the sustained release form be performed strictly in accordance with the instructions given in section 6.6.
Following reconstitution, the suspension has to be injected immediately.
Dosage and method of administration
The dosage of one syringe, equivalent to 3.75 mg triptorelin, is injected every 28 days either subcutaneously (e.g. into the skin of the abdomen, the buttock or thigh) or deep intramuscularly. The injection site should be changed each time.
Men:
Once every four weeks an injection with one syringe, equivalent to 3.75 mg triptorelin. In order to continually suppress testosterone levels, it is important to comply with a 4-weekly administration.
Women:
- Uterine myomas and endometriosis:
Once every four weeks an injection with one syringe, equivalent to 3.75 mg triptorelin. The treatment must be initiated in the first 5 days of the cycle.
Children:
Dosing at the beginning of treatment should be based on body weight, one injection of triptorelin should be injected on days 0, 14, and 28. Thereafter one injection every 4 weeks. Should the effect be insufficient, the injections may be given every 3 weeks. Dosing should be based on body weight according to the table.
|
Body weight |
Dosing |
|
< 20 kg |
1.875 mg (half dose) |
|
20 - 30 kg |
2.5 mg (2/3 dose) |
|
> 30 kg |
3.75 mg (full dose) |
Note for specific patient groups:
- There is no need to adjust the dose for the elderly.
- According to current data, dose reduction or prolongation of the dosage interval in patients with impaired renal function is not necessary.
Duration of administration
- Prostate carcinoma:
Treatment with GONAPEPTYL Depot is usually a long-term therapy.
- Uterine myomas and endometriosis:
The duration of treatment depends on the initial degree of severity of endometriosis and on the evolution of its clinical manifestations (functional and anatomical) and on the evolution of the volume of the uterine myomas, determined by ultrasonography during treatment. Normally, the maximum attainable result is achieved after 3 to 4 injections.
In view of the possible effect on bone density, therapy should not exceed a duration of 6 months (see 4.4).
- Central precocious puberty (CPP) :
Treatment should be stopped if a bone maturation of older than 12 years in girls and older than 13 years in boys has been achieved.
4.3 Contraindications
General:
Known hypersensitivity to triptorelin, poly-(d,l lactide coglycolide), dextran, or to any of the excipients.
Hypersensitivity to gonadotrophin-releasing hormone (GnRH) or any other GnRH analogue.
In women:
- Pregnancy
- Lactation period
4.4 Special warnings and precautions for use
General:
The use of GnRH agonists may cause reduction in bone mineral density. In men, preliminary data suggest that the use of a bisphosphonate in combination with a GnRH agonist may reduce bone mineral loss.
Particular caution is necessary in patients with additional risk factors for osteoporosis (e.g. chronic alcohol abuse, smokers, long-term therapy with drugs that reduce bone mineral density, e.g. anticonvulsants or corticoids, family history of osteoporosis, malnutrition).
Rarely, treatment with GnRH agonists may reveal the presence of a previously unknown gonadotroph cell pituitary adenoma. These patients may present with a pituitary apoplexy characterised by sudden headache, vomiting, visual impairment and ophthalmoplegia.
There is an increased risk of incident depression (which may be severe) in patients undergoing treatment with GnRH agonists, such as triptorelin. Patients should be informed accordingly and treated as appropriate if symptoms occur.
Mood changes have been reported. Patients with known depression should be monitored closely during therapy.
Men:
Initially, triptorelin, like other GnRH agonists, causes a transient increase in serum testosterone levels. As a consequence, isolated cases of transient worsening of signs and symptoms of prostate cancer may occasionally develop during the first weeks of treatment. During the initial phase of treatment, consideration should be given to the additional administration of a suitable anti-androgen to counteract the initial rise in serum testosterone levels and the worsening of clinical symptoms.
A small number of patients may experience a temporary worsening of signs and symptoms of their prostate cancer (tumour flare) and temporary increase in cancer related pain (metastatic pain), which can be managed symptomatically.
As with other GnRH agonists, isolated cases of spinal cord compression or urethral obstruction have been observed. If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy (surgical castration) should be considered. Careful monitoring is indicated during the first weeks of treatment, particularly in patients suffering from vertebral metastasis, at the risk of spinal cord compression, and in patients with urinary tract obstruction.
After surgical castration, triptorelin does not induce any further decrease in serum testosterone levels.
Long-term androgen deprivation either by bilateral orchiectomy or administration of GnRH analogues is associated with increased risk of bone loss and may lead to osteoporosis and increased risk of bone fracture.
Androgen deprivation therapy may prolong the QT interval.
In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval (see section 4.5) physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating GONAPEPTYL,
In addition, from epidemiological data, it has been observed that patients may experience metabolic changes (e.g. glucose intolerance), or an increased risk of cardiovascular disease during androgen deprivation therapy. However, prospective data did not confirm the link between treatment with GnRH analogues and an increase in cardiovascular mortality. Patients at high risk for metabolic or cardiovascular diseases should be carefully assessed before commencing treatment and adequately monitored during androgen deprivation therapy.
Administration of triptorelin in therapeutic doses result in suppression of the pituitary gonadal system. Normal function is usually restored after treatment is discontinued. Diagnostic tests of pituitary gonadal function conducted during treatment and after discontinuation of therapy with GnRH analogues may therefore be misleading.
Women:
Gonapeptyl Depot should only be prescribed after careful diagnosis (e.g. laparoscopy).
It should be confirmed that the patient is not pregnant before prescription of triptorelin.
Since menses should stop during GONAPEPTYL Depot treatment, the patient should be instructed to notify her physician if regular menstruation persists.
Loss of bone mineral density
The use of GnRH agonists is likely to cause reduction in bone mineral density averaging 1% per month during a six month treatment period. Every 10% reduction in bone mineral density is linked with about a two to three times increased fracture risk. For this reason, therapy without add back treatment should not exceed a duration of 6 months. After withdrawal of treatment, the bone loss is generally reversible within 6 - 9 months.
In the majority of women, currently available data suggest that recovery of bone loss occurs after cessation of therapy.
No specific data is available for patients with established osteoporosis or with risk factors for osteoporosis (e.g. chronic alcohol abuses, smokers, long-term therapy with drugs that reduce bone mineral density, e.g. anticonvulsants or corticoids, family history of osteoporosis, malnutrition, e.g. anorexia nervosa). Since reduction in bone mineral density is likely to be more detrimental in these patients, treatment with triptorelin should be considered on an individual basis and only be initiated if the benefits of treatment outweigh the risk following a very careful appraisal. Consideration should be given to additional measures in order to counteract loss of bone mineral density.
Uterine myomas and endometriosis:
A supervening metrorrhagia in the course of treatment is abnormal (apart from the first month), and should lead to verification of plasma oestrogen level. Should this level be less than 50 pg/ml, possible associated organic lesions should be sought. After withdrawal of treatment, ovarian function resumes, e.g. menstrual bleeding will resume after 7-12 weeks after the final injection.
Non-hormonal contraception should be used during the initial month of treatment as ovulation may be triggered by the initial release of gonadotropins. It should also be used from 4 weeks after the last injection until resumption of menstruation or until another contraceptive method has been established.
During treatment of uterine myomas the size of uterus and myoma should be determined regularly, e.g. by means of ultrasonography. Disproportionally fast reduction of uterus size in comparison with the reduction of myoma tissue has in isolated cases led to bleeding and sepsis. There have been a few reports of bleeding in patients with submucous fibroids following GnRH analogue therapy. Typically the bleeding has occurred 6 - 10 weeks after the initiation of therapy.
Children:
The chronological age at the beginning of therapy should be under 9 years in girls and under 10 years in boys.
In girls initial ovarian stimulation at treatment initiation, followed by the treatment-induced oestrogen withdrawal, may lead, in the first month, to vaginal bleeding of mild or moderate intensity.
After finalising the therapy, development of puberty characteristics will occur. Information with regards to future fertility is still limited. In most girls menses will start on average one year after ending the therapy, which in most cases is regular.
Bone mineral density may decrease during GnRH therapy for central precocious puberty. However, after cessation of treatment subsequent bone mass accrual is preserved and peak bone mass in late adolescence does not seem to be affected by treatment.
Slipped capital femoral epiphysis can be seen after withdrawal of GnRH treatment. The suggested theory is that the low concentrations of oestrogen during treatment with GnRH agonists weakens the epiphyseal plate. The
increase in growth velocity after stopping the treatment subsequently results in a reduction of the shearing force needed for displacement of the epiphysis.
The treatment of children with progressive brain tumours should follow a careful individual appraisal of the risks and benefits.
Pseudo-precocious puberty (gonadal or adrenal tumour or hyperplasia) and gonadotropin-independent precocious puberty (testicular toxicosis, familial Leydig cell hyperplasia) should be precluded.
Allergic and anaphylactic reactions have been reported in adults and children. These include both local site reactions and systemic symptoms. The pathogenesis could not be elucidated. A higher reporting rate was seen in children.
4.5 Interaction with other medicinal products and other forms of interaction
When triptorelin is co-administered with drugs affecting pituitary secretion of gonadotropins caution should be given and it is recommended that the patient’s hormonal status should be supervised.
Since androgen deprivation treatment may prolong the QT interval, the concomitant use of GONAPEPTYL with medicinal products known to prolong the QT interval or medicinal products able to induce Torsade de pointes such as class IA (e.g. quinidine, disopyramide) or class III (e.g. amiodarone, sotalol, dofetilide, ibutilide) antiarrhythmic medicinal products, methadone, moxifloxacin, antipsychotics, etc. should be carefully evaluated (see section 4.4).
No formal drug-drug interaction studies have been performed. The possibility of interactions with commonly used medicinal products, including histamine liberating products, cannot be excluded.
4.6 Pregnancy and lactation
Prior to treatment, potentially fertile women should be examined carefully to exclude pregnancy.
Very limited data on the use of triptorelin during pregnancy do not indicate an increased risk of congenital malformations. However, long-term follow-up studies on development are far too limited. Animal data do not indicate direct or indirect harmful effects with respect to pregnancies or postnatal developments, but there are indications for foetotoxicity and delayed parturition. Based on the pharmacological effects disadvantageous influence on the pregnancy and the offspring cannot be excluded and GONAPEPTYL Depot should not be used during pregnancy.
Women of childbearing potential should use effective non-hormonal contraception during therapy until menses resume.
It is not known whether triptorelin is excreted in human milk. Because of the potential for adverse reactions from triptorelin in nursing infants, breastfeeding should be discontinued prior to and throughout administration.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, the ability to drive and use machines may be impaired should the patient experience dizziness, somnolence and visual disturbances being possible undesirable effects of treatment, or resulting from the underlying disease
4.8 Undesirable effects
Adverse experiences reported among patients treated with triptorelin during clinical trials and from post-marketing surveillance are shown below. As a consequence of decreased testosterone or oestrogen levels, most patients are expected to experience adverse reactions, with hot flushes being the most frequently reported (30% in men and 75-100% in women). Additionally, impotence and decreased libido should be expected in 30-40% of male patients, while bleeding/spotting, sweating, vaginal dryness and/or dyspareunia, decrease in libido, headache and mood changes are expected in more than 10% of women.
Due to the fact that the testosterone levels normally increase during the first week of treatment, worsening of symptoms and complaints may occur (e.g. urinary obstruction, skeletal pain due to metastases, compression of the spinal cord, muscular fatigue and lymphatic oedema of the legs). In some cases urinary tract obstruction decreases the kidney function. Neurological compression with asthenia and paraesthesia in the legs has been observed.
General tolerance in men (refer to Special Warnings and Precautions for use) As seen with other GnRH agonist therapies or after surgical castration, the most commonly observed adverse events related to triptorelin treatment were due to its expected pharmacological effects: Initial increase in testosterone levels, followed by almost complete suppression of testosterone. These effects included hot flushes (50%), erectile dysfunction and decreased libido.
The following adverse reactions, considered as at least possibly related to triptorelin treatment, were reported. Most of these are known to be related to biochemical or surgical castration.
|
MedDRA |
Very |
Common |
Uncommon |
Not known |
|
System |
common |
( 1/100 to |
( 1/1000 to | |
|
Organ Class |
( 1/10) |
<1/10) |
<1/100) |
|
Men | ||||
|
Infections and infestations |
Nasopharyngitis | |||
|
Immune system disorders |
Hypersensitivity |
Anaphylactic reaction | ||
|
Metabolism and nutrition disorders |
Decreased appetite |
Increased appetite, gout, diabetes mellitus | ||
|
Psychiatric disorders |
Libido decreased |
Mood changes, depressed mood, depression, sleep disorder |
Insomnia, confusional state, decreased activity, euphoric mood, anxiety, loss of libido | |
|
Nervous system disorder |
Headache |
Dizziness, paraesthesia, memory impairment, dysgeusia, somnolence, dysstasia | ||
|
Eye disorders |
Abnormal sensation in eye, visual impairment, vision blurred | |||
|
Ear and labyrinth disorders |
Tinnitus, vertigo | |||
|
Vascular disorders |
Hot flushes |
Embolism, hypertension |
Hypotension | |
|
Respiratory, thoracic and mediastinal disorders |
Asthma aggravated |
Dyspnoea, orthopnoea, epistaxis | ||
|
Gastrointesti nal disorders |
Nausea |
Abdominal pain upper, dry mouth |
Abdominal pain, constipation, diarrhoea, vomiting, abdominal distension, flatulence, gastralgia | |
|
Skin and subcutaneous tissue disorders |
Hyperhidrosis |
Hypotrichosi s, alopecia |
Acne, pruritus, rash, blister, angioedema, urticaria, purpura | |
|
Musculoskel etal and connective tissue disorders |
Bone pain |
Myalgia, arthralgia |
Back pain, musculoskeletal pain, pain in extremity, muscle spasms, muscular weakness, joint stiffness, joint swelling, musculoskeletal stiffness, osteoarthritis | |
|
Renal and urinary disorders |
Dysuria | |||
|
Reproductive system and breast disorders |
Erectile dysfunction |
Gynaecomastia |
Testicular atrophy |
Breast pain, testicular pain, ejaculation failure |
|
General disorders and administratio n site conditions |
Fatigue, injection site reaction, injection site pain, irritability |
Asthenia, injection site erythema, injection site inflammation, oedema, pain, chills, chest pain, influenza like illness, pyrexia, malaise | ||
|
Investigation s |
Blood lactate dehydrogena se increased, gamma- glutamyltran sferase increased, aspartate aminotransfe rase increased, alanine aminotransfe rase increased, weight increased, weight |
Blood creatinine increased, blood pressure increased, blood urea increased, blood alkaline phosphatase increased, body temperature increased QT prolongation (see section 4.4 and 4.5) |
|
decreased | ||||
|
Triptorelin causes a transient increase in circu |
ating testosterone levels within | |||
the first week after the initial injection of the sustained release formulation. With this initial increase in circulating testosterone levels, a small percentage of patients (< 5%) may experience a temporary worsening of signs and symptoms of their prostate cancer (tumour flare), usually manifested by an increase in urinary symptoms (< 2%) and metastatic pain (5%), which can be managed symptomatically. These symptoms are transient and usually disappear in one to two weeks.
Isolated cases of exacerbation of disease symptoms, either urethral obstruction or spinal cord compression by metastasis have occurred. Therefore, patients with metastatic vertebral lesions and/or with upper or lower urinary tract obstruction should be closely observed during the first few weeks of therapy (see Special warnings and special precautions for use).
The use of GnRH agonists, to treat prostate cancer may be associated with increased bone loss and may lead to osteoporosis and increases the risk of bone fracture.
General tolerance in women (refer to Special Warnings and Precautions for use)
As a consequence of decreased oestrogen levels, the most commonly reported adverse events (expected in 10% of women or more) were headache, libido decreased, sleep disorder, mood changes, dyspareunia, dysmenorrhoea, genital haemorrhage, ovarian hyperstimulation syndrome, ovarian hypertrophy pelvic pain, abdominal pain, vulvovaginal dryness, hyperhidrosis, hot flushes and asthenia.
The following adverse reactions, considered as at least possibly related to triptorelin treatment, were reported. Most of these are known to be related to biochemical or surgical castration.
|
MedDRA System Organ Class |
Very common ( 1/10) |
Common ( 1/100 to <1/10) |
Uncommon ( 1/1000 to <1/100) |
Not known | ||
|
Women | ||||||
|
Immune system disorders |
Hypersensitivity |
Anaphylactic reaction | ||||
|
Psychiatric disorders |
Libido decreased, mood changes, sleep disorder |
Depressed mood, depression |
Confusional state, anxiety | |
|
Nervous system disorder |
Headache |
Paraesthesia |
Dizziness | |
|
Eye disorders |
Visual impairment |
Vision blurred | ||
|
Ear and labyrinth disorders |
Vertigo | |||
|
Vascular disorders |
Hot flushes | |||
|
Respiratory, thoracic and mediastinal disorders |
Dyspnoea | |||
|
Gastrointestinal disorders |
Abdominal pain |
Nausea |
Abdominal discomfort, diarrhoea, vomiting | |
|
Skin and subcutaneous tissue disorders |
Hyperhidrosis |
Pruritus, rash, angioedema, urticaria | ||
|
Musculoskeletal and connective tissue disorders |
Bone pain |
Myalgia, arthralgia |
Back pain |
Bone disorder(*), muscle spasms, muscular weakness |
|
Reproductive system and breast disorders |
Vaginal haemorrhage, vulvovaginal dryness, dyspareunia, dysmenorrhoea , ovarian hyperstimulati on syndrome ovarian hypertrophy, pelvic pain |
Breast pain, menorrhagia, metrorrhagia, amenorrhoea, | ||
|
General disorders and administration site conditions |
Asthenia |
Fatigue, injection site reaction, injection site pain, irritability |
Injection site erythema, injection site inflammation, pyrexia, malaise |
|
Investigations |
Blood lactate |
Blood | ||
|
dehydrogenase |
pressure | |||
|
increased, |
increased, | |||
|
gamma- |
weight | |||
|
glutamyltransfera |
increased, | |||
|
se increased, |
weight | |||
|
aspartate aminotransferase increased, alanine aminotransferase increased, blood cholesterol increased |
decreased |
(*) Slight trabecular bone loss may occur. This is generally reversible within 6-9 months after treatment discontinuation (see section 4.4).
At the beginning of treatment, the symptoms of endometriosis including pelvic pain, dysmenorrhoea may be exacerbated very commonly (> 10%) during the initial transient increase in plasma oestradiol levels. These symptoms are transient and usually disappear in one or two weeks.
Genital haemorrhage including menorrhagia, metrorrhagia may occur in the month following the first injection.
Ovarian hypertrophy, pelvic and/or abdominal pain may be observed.
General tolerance in children (refer to Special Warnings and Precautions for use)
|
MedDRA System Organ Class |
Very common ( 1/10) |
Common ( 1/100 to <1/10) |
Uncommon ( 1/1000 to <1/100) |
Not known |
|
Children | ||||
|
Immune system disorders |
Anaphylactic reaction |
Hypersensitivity reaction | ||
|
Psychiatric disorders |
Mood changes, depression |
Affect lability, nervousness | ||
|
Nervous system disorder |
Headache | |||
|
Eye disorders |
Vision blurred, Visual impairment | |||
|
Vascular disorders |
Hot flushes | |||
|
Respiratory, thoracic and mediastinal disorders |
Epistaxis | |||
|
Gastrointestinal disorders |
Nausea, vomiting |
Abdominal discomfort, abdominal pain | ||
|
Skin and subcutaneous tissue disorders |
Rash, angioneurotic edema, urticaria, alopecia, erythema | |||
|
Musculoskeletal and connective tissue disorders |
Epiphysiolysis (*), myalgia | |||
|
Reproductive system and breast disorders |
Vaginal haemorrhage, vaginal discharge |
Genital haemorrhage | ||
|
General disorders and administration site conditions |
Injection site erythema, injection site inflammation, malaise, pain, injection site pain | |||
|
Investigations |
Blood pressure increased, weight increased |
(*) A few cases of slipped capital femoral epiphysis have been reported during use with triptorelin.
Cases of pre-existing pituitary adenomas enlargement were reported during treatment with LH-RH agonists, however it has not yet been observed with triptorelin therapy.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
There is insufficient experience of overdosing with triptorelin to draw conclusions on possible adverse effects. Considering the package form and the pharmaceutical form, overdosing is not expected.
If overdose occurs, symptomatic management is indicated.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Gonadorelinanaloga ATC code: L02AE04
Triptorelin is a synthetic decapeptide analogue of the natural gonadotrophinreleasing hormone (GnRH). GnRH is a decapeptide, which is synthesised in the hypothalamus and regulates the biosynthesis and release of the gonadotrophins LH (luteinising hormone) and FSH (follicle stimulating hormone) by the pituitary. Triptorelin stimulates the pituitary more strongly to secretion of LH and FSH than a comparable dose of gonadorelin, whereas the duration of action is longer. The increase of LH and FSH levels will initially lead to an increase of serum testosterone concentrations in men or serum estrogen concentrations in women. Chronic administration of a GnRH agonist results in an inhibition of pituitary LH- and FSH-secretion. This inhibition leads to a reduction in steroidogenesis, by which the serum estradiol concentration in women and the serum testosterone concentration in men fall to within the postmenopausal or castrate range, respectively, i.e. a hypogonadotrophic hypogonadal state. In children with precocious puberty, the concentration of estradiol or testosterone will decrease to within the prepubertal range. Plasma DHEAS (dihydroepiandrostenedion sulphate) levels are not influenced. Therapeutically, this leads to a decrease in growth of testosterone-sensitive prostate tumours in men, and to reduction of endometriosis foci and estrogen-dependent uterus myomas in women. Regarding uterine myoma, maximal benefit of treatment is observed in women with anemia (hemoglobin inferior or equal to 8 g/dl). In children suffering from CPP triptorelin treatment leads to a suppression of the secretion of gonadotropins, estradiol, and testosterone to prepubertal levels. This results in arrest or even regression of pubertal signs and an increase in adult height prediction in CPP patients.
5.2 Pharmacokinetic properties
After intramuscular administration of GONAPEPTYL Depot, the plasma concentrations of triptorelin are determined by the (slow) degradation of the poly-(d,l lactide coglycolide) polymer. The mechanism inherent to this administration form enables this slow release of triptorelin from the polymer.
After I.M. or S.C. application of a triptorelin depot-formulation (sustained-release microcapsules), a rapid increase in the concentration of triptorelin in plasma is recorded, with a maximum in the first hours. Then the triptorelin concentration declines notably within 24 hours. On day 4 the value reaches a second maximum, falling below the detection limit in a biexponential course after 44 days. After S.C. injections the triptorelin increase is more gradual and in a somewhat lower concentration than after I.M. injections. After S.C. injection, the decline in the triptorelin concentration takes longer, with values falling below the detection limit after 65 days.
During treatment over a period of 6 months and an administration every 28 days, there was no evidence of triptorelin accumulation in both modes of administration. Plasma triptorelin values decreased to approx. 100 pg/ml before the next application after I.M. or S.C. application (median values). It is to be assumed that the non-systemically available proportion of triptorelin is metabolized at the injection site, e.g. by macrophages.
In the pituitary, the systemically available triptorelin is inactivated by N-terminal cleavage via pyroglutamyl-peptidase and a neutral endopeptidase. In the liver and the kidneys, triptorelin is degraded to biologically inactive peptides and amino acids.
40 minutes after the end of an infusion of 100 |ig triptorelin (over 1 hour) 314% of the administered dose has already been eliminated by the kidney.
For patients with an impaired renal function, adaptation and individualization of therapy with the triptorelin depot-formulation seems to be unnecessary, on account of the subordinate significance of the renal elimination route and the broad therapeutic range of triptorelin as an active component.
Bioavailability:
Men:
The systemic bioavailability of the active component triptorelin from the intramuscular depot is 38.3% in the first 13 days. Further release is linear at
0.92% of the dose per day on average. Bioavailability after S.C. application is 69% of I.M. availability.
Women:
After 27 test days, 35.7% of the applied dose can be detected on average, with 25.5% being released in the first 13 days and further release being linear at 0.73% of the dose per day on average.
General:
Calculation of the model-depending kinetic parameters (t'A, Kel, etc.) is inapplicable in presentations with a strongly protracted release of the active component.
5.3 Preclinical safety data
In rats, but not in mice treated over a long period of time with triptorelin, an increase in pituitary tumors has been detected. The influence of triptorelin on pituitary abnormalities in humans is unknown. The observation is considered not to be relevant to humans. Pituitary tumors in rodents in connection with other LHRH analogues have also been known to occur. Triptorelin has been shown to be embryo-/foetotoxic and to cause a delay in embryo-foetal development as well as delay in parturition in rats. Preclinical data reveal no special hazard to humans based on repeat dose toxicity and genotoxicity studies. Single I.M. or S.C. injection of GONAPEPTYL Depot or its suspension agent produced delayed foreign body reactions at the injection site. Within 8 weeks, these late reactions were nearly reversed after I.M. injection but only slightly reversed after S.C. injection. Local tolerance of Gonapeptyl Depot after I.V. injection was limited
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
One pre-filled syringe with powder contains:
Poly-(d,l lactide coglycolide)
Propylene glycol dicaprylocaprate
One pre-filled syringe with one ml suspension agent contains: Dextran 70 Polysorbate 80 Sodium chloride
Sodium hydrogen phosphate dihydrate Sodium hydroxide Water for injection
6.2 Incompatibilities
In the absence of compatibility studies this medicinal product should not be mixed with other medicinal products.
6.3 Shelf life
3 years
Reconstituted suspension: 3 minutes
6.4
Special precautions for storage
Store at 2°C - 8°C (in a refrigerator). Keep the container in the outer carton.
6.5 Nature and contents of container
Powder: Pre-filled syringe Solvent: Pre-filled syringe
Pre-filled syringes (borosilicate glass type I, clear) with a connector (polypropylene), black chlorobutyl rubber stopper (plunger stopper, type I) and injection needle.
Pack sizes:
1 pre-filled syringe (powder) plus 1 pre-filled syringe (solvent)
3 pre-filled syringes (powder) plus 3 pre-filled syringes (solvent)
6.6 Special precautions for disposal
GONAPEPTYL Depot is for single use only and any unused suspension
should be discarded.
1. Preparation
Instructions for the physician how to prepare the suspension.
Since successful treatment depends upon correct preparation of the suspension,
the following instructions must be strictly followed.
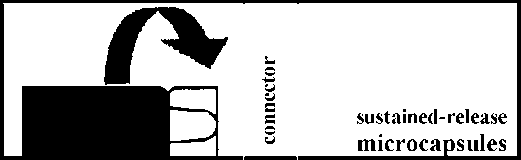
• Take the package of GONAPEPTYL Depot from the refrigerator.
• Remove the cap from the disposable syringe containing the powder. Keep upright to prevent spilling.
• Open the package with the connector without removing the connector.
• Screw the syringe containing the sustained release microparticles on the connector in the package, then remove it.
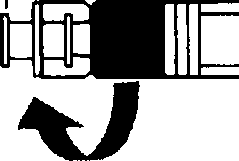
• Screw the syringe containing the suspension agent tightly on the free end of the connector and ensure that it fits tightly,
suspension agent
Preparation
2. Reconstitution of a suspension
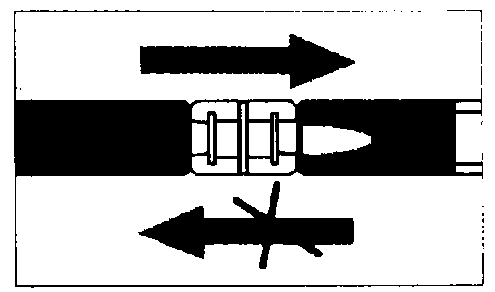
Empty the liquid into the syringe with the powder, then shoot it back and forth into the first syringe - the first two or three times without pushing the injection rod all the way in. Repeat this about 10 times or until you have a homogeneous milky white to faintly yellow suspension. While preparing the suspension, you might possibly create some foam. It is important that the foam be dissolved or removed from the syringe before giving the injection.
Mixing
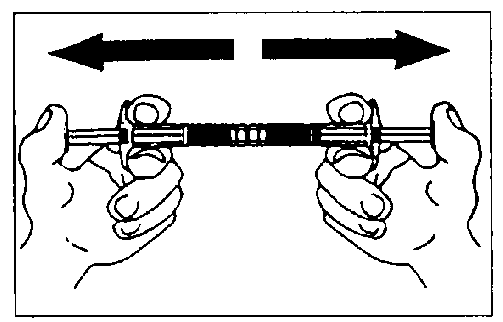
Mix approximately 10 times
3. Injection
• Remove the connector together with the empty syringe.
• Mount the injection needle on the syringe with the ready-to-use suspension.
Inject subcutaneously or deep into the muscle immediately.
7 MARKETING AUTHORISATION HOLDER
Ferring Pharmaceuticals Ltd Drayton Hall
Church Road
West Drayton
UB7 7PS
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 03194/0085
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
07/08/2006
10 DATE OF REVISION OF THE TEXT
07/05/2015