Hemosol B0 Solution For Haemofiltration And Haemodialysis
UK IE MT Package Leaflet: Information for user
Hemosol B0 solution for haemodialysis/haemofiltration Sodium chloride/ Calcium chloride dihydrate/ Magnesium chloride hexahydrate/ Lactic acid/ Sodium hydrogen carbonate Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Hemosol B0 is and what it is used for
2. What you need to know before you use Hemosol B0
3. How to use Hemosol B0
4. Possible side effects
5. How to store Hemosol B0
6. Contents of the pack and other information
1. What Hemosol B0 is and what it is used for
Hemosol B0 is used in hospitals in intensive care treatments to correct chemical imbalance of the blood which is caused by kidney failure. The treatments are designed to remove accumulated waste products from the blood when the kidneys are not functioning.
Hemosol B0 is used in the following types of treatment in adult and children of all ages:
• haemofiltration,
• haemodiafiltration and
• haemodialysis.
2. What you need
to know before you use Hemosol B0
Do not use Hemosol B0 in the following cases:
There are no symptoms or conditions that make Hemosol B0 unusable.
I 2 |
Take special care with Hemosol B0
As the Hemosol B0 is potassium-free, special attention will be given to the level of potassium in your blood. Should you suffer from low potassium a potassium supplement might be necessary.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of salts in the blood (electrolytes) will be monitored.
Warnings and precautions
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only.
They will ensure a safe use of the medicine.
Children
There are no specific warnings and precautions when using this medicine for children.
Other medicines and Hemosol B0
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
This is because the concentration level in the blood of some of other medicines being taken may be reduced during the treatment with Hemosol B0. Your doctor will decide if other medicines being taken should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions);
as the risk of irregular or rapid beating of the heart (cardiac arrhythmia) caused by digitalis is increased during a low concentration of potassium in the blood (hypokalaemia).
• Vitamin D and medicinal products containing calcium, as they can increase the risk of a high concentration of calcium in the blood (hypercalcaemia).
• Any addition of sodium bicarbonate as it may increase the risk of excess of bicarbonate in your blood (metabolic alkalosis).
Hemosol B0 D05759001 Rev. 2015-02/1 REG 3857
Pregnancy, breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. No effects on fertility or during pregnancy or on the breast-fed newborn/infant are anticipated.
Your doctor will decide if you should be given Hemosol B0 if you are pregnant or breast-feeding.
Driving and using machines
Hemosol B0 will not have any effect on your ability to drive or use machines.
3. How to use Hemosol B0
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only.
The volume of Hemosol B0, and therefore the dose used, will depend on your condition. The dose volume will be determined by the physician responsible for your treatment.
Hemosol B0 can be administered directly into the bloodstream (intravenously) or via haemodialysis, where the solution flows on one side of a dialysis membrane while the blood flows on the other side.
If you use more Hemosol B0 than you should
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only and your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Therefore, it is unlikely that you will use more Hemosol B0 than you should.
In the unlikely event that an overdose occurs, your doctor will take the necessary corrective measures and adjust your dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
There are some undesirable effects which can be caused by the dialysis treatment, such as nausea, vomiting, muscle cramps, low blood pressure (hypotension) and changes of levels of salt in the blood (electrolyte disturbances).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via
Malta
ADR Reporting Website:
www.medicinesauthority.gov.mt/
adrporta
Republic of Ireland:
HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafety@hpra.ie
United Kingdom:
Yellow Card Scheme Website:
By reporting side effects you can help provide more information on the safety of this medicine.
6. Contents of the pack and other information
What Hemosol B0 contains
The active substances before and after reconstitution are shown below.
Active substances before reconstitution:
1000 ml of solution from the small compartment (A) contains: Calcium chloride, 2 H2O 5.145 g Magnesium chloride, 6 H2O 2.033 g Lactic acid 5.4 g
1000 ml of solution from the large compartment (B) contains: Sodium hydrogen carbonate 3.09 g Sodium chloride 6.45 g
Active substances after reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) which composition is:
mmol/l
Theoretical Osmolarity:
287 mOsm/l
Manufacturer:
Gambro Dasco S.p.A.
Via Stelvio, 94 23035 Sondalo (SO)
Italy
This medicinal product is authorised in the Member States of the EEA under the following names: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom: Hemosol B0.
Hungary: Hemosol kaliummentes.
This leaflet was last revised in 02/2015.
5. How to store Hemosol B0
Keep this medicine out of the sight and reach of children Do not use this medicine after the expiry date which is stated on the back of the bag and the box label after EXP. The expiry date refers to the last day of that month.
Do not store below +4°C.
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a microbiological point of view, the reconstituted solution should be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and should not be longer than 24 hours including the duration of the treatment.
The other ingredients are: carbon dioxide and water for injections.
What Hemosol B0 looks like and contents of the pack
Hemosol B0 is presented in a two-compartment bag. The bag is over wrapped with a transparent film. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions.
The reconstituted solution is clear and colourless. Each bag (A+B) contains 5000 ml solution for haemofiltration, haemodiafiltration and/ or haemodialysis.
Each box contains two bags and one package leaflet.
Marketing Authorisation Holder
Gambro Lundia AB Magistratsvagen 16 SE-226 43 Lund Sweden
I 3 I
Hemosol B0 D05759001 Rev. 2015-02/1 REG 3857
Hemosol B0 solution for haemodialysis/haemofiltration
Carefully follow the instructions for use/ handling.
Solution A must be mixed with solution B before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
If heating of the solution to body temperature (+37°C) is necessary the procedure must be carefully controlled verifying that the solution is clear and without particles. Additional sodium bicarbonate substitution may increase the risk of metabolic alkalosis.
Before and during treatment, haemodynamic status, fluid balance, electrolyte and acid-base balance should be closely monitored. As Hemosol B0 is potassium-free, special attention should be given to potassium levels. Phosphate and potassium supplement might be necessary.
The volume of Hemosol B0 used will depend on the patient's clinical condition and the target fluid balance. Continued application of haemofiltration will remove excess fluid and electrolytes.
I 8 |
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored:
• In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced.
• In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
Overdose will result in fluid overload if the patient is suffering from renal failure, and it could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
The use of contaminated haemofiltration solution may cause sepsis and shock.
Instructions for use / Handling
Do not use with a haemodialysis monitor. You should only use monitors for Continuous Renal Replacement Therapies.
The solution is packaged in a two compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Hemosol B0 D05759001 Rev. 2015-02/1 REG 3857
Use only if the solution is clear and the over wrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution.
It is the responsibility of the physician to judge the compatibility of an additive medication with Hemosol B0 by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water within the pH limits of Hemosol B0 (pH limits of reconstituted solution is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
X...............................................................................................................................................................................................................................................................................................................................X
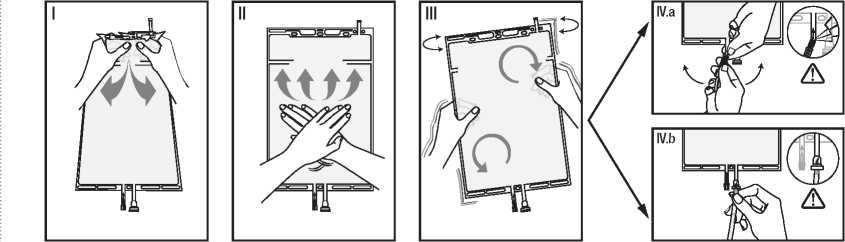
I Remove the over wrap from the bag immediately before use and discard any other packaging materials. Open the seal by holding the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Secure complete mixing of the solution by shaking the bag gently. The solution is now ready for use and the bag can be hung on the equipment.
(See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag; tighten. Using thumb and fingers, break the coloured frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure IV.a below)
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below).
The solution should be used immediately after removal of the over wrap and after addition of solution A to solution B. If not used immediately, the reconstituted solution should be used within 24 hours, including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
o
Hemosol B0 D05759001 Rev. 2015-02/1 REG 3857
I 9 I
Hemosol B0 solution for haemodialysis/haemofiltration Sodium chloride/ Calcium chloride dihydrate/ Magnesium chloride hexahydrate/ Lactic acid/ Sodium hydrogen carbonate Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Hemosol B0 is and what it is used for
2. What you need to know before you use Hemosol B0
3. How to use Hemosol B0
4. Possible side effects
5. How to store Hemosol B0
6. Contents of the pack and other information
1. What Hemosol B0 is and what it is used for
Hemosol B0 is used in hospitals in intensive care treatments to correct chemical imbalance of the blood which is caused by kidney failure. The treatments are designed to remove accumulated waste products from the blood when the kidneys are not functioning.
Hemosol B0 is used in the following types of treatment in adult and children of all ages:
• haemofiltration,
• haemodiafiltration and
• haemodialysis.
2. What you need
to know before you use Hemosol B0
Do not use Hemosol B0 in the following cases:
There are no symptoms or conditions that make Hemosol B0 unusable.
I 2 |
Take special care with Hemosol B0
As the Hemosol B0 is potassium-free, special attention will be given to the level of potassium in your blood. Should you suffer from low potassium a potassium supplement might be necessary.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of salts in the blood (electrolytes) will be monitored.
Warnings and precautions
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only.
They will ensure a safe use of the medicine.
Children
There are no specific warnings and precautions when using this medicine for children.
Other medicines and Hemosol B0
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
This is because the concentration level in the blood of some of other medicines being taken may be reduced during the treatment with Hemosol B0. Your doctor will decide if other medicines being taken should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions);
as the risk of irregular or rapid beating of the heart (cardiac arrhythmia) caused by digitalis is increased during a low concentration of potassium in the blood (hypokalaemia).
• Vitamin D and medicinal products containing calcium, as they can increase the risk of a high concentration of calcium in the blood (hypercalcaemia).
• Any addition of sodium bicarbonate as it may increase the risk of excess of bicarbonate in your blood (metabolic alkalosis).
Hemosol B0 D05787001 Rev. 2015-02/1 REG 4741
Pregnancy, breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. No effects on fertility or during pregnancy or on the breast-fed newborn/infant are anticipated.
Your doctor will decide if you should be given Hemosol B0 if you are pregnant or breast-feeding.
Driving and using machines
Hemosol B0 will not have any effect on your ability to drive or use machines.
3. How to use Hemosol B0
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only.
The volume of Hemosol B0, and therefore the dose used, will depend on your condition. The dose volume will be determined by the physician responsible for your treatment.
Hemosol B0 can be administered directly into the bloodstream (intravenously) or via haemodialysis, where the solution flows on one side of a dialysis membrane while the blood flows on the other side.
If you use more Hemosol B0 than you should
Hemosol B0 is a product to be used in hospitals and administered by medical professionals only and your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Therefore, it is unlikely that you will use more Hemosol B0 than you should.
In the unlikely event that an overdose occurs, your doctor will take the necessary corrective measures and adjust your dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
There are some undesirable effects which can be caused by the dialysis treatment, such as nausea, vomiting, muscle cramps, low blood pressure (hypotension) and changes of levels of salt in the blood (electrolyte disturbances).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via
Malta
ADR Reporting Website:
www.medicinesauthority.gov.mt/
adrporta
Republic of Ireland:
HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafety@hpra.ie
United Kingdom:
Yellow Card Scheme Website:
By reporting side effects you can help provide more information on the safety of this medicine.
6. Contents of the pack and other information
What Hemosol B0 contains
The active substances before and after reconstitution are shown below.
Active substances before reconstitution:
1000 ml of solution from the small compartment (A) contains: Calcium chloride, 2 H2O 5.145 g Magnesium chloride, 6 H2O 2.033 g Lactic acid 5.4 g
1000 ml of solution from the large compartment (B) contains: Sodium hydrogen carbonate 3.09 g Sodium chloride 6.45 g
Active substances after reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) which composition is:
mmol/l
Theoretical Osmolarity:
287 mOsm/l
Manufacturer:
Gambro Dasco S.p.A.
Via Stelvio, 94 23035 Sondalo (SO)
Italy
This medicinal product is authorised in the Member States of the EEA under the following names: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom: Hemosol B0.
Hungary: Hemosol kaliummentes.
This leaflet was last revised in 02/2015.
5. How to store Hemosol B0
Keep this medicine out of the sight and reach of children Do not use this medicine after the expiry date which is stated on the back of the bag and the box label after EXP. The expiry date refers to the last day of that month.
Do not store below +4°C.
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a microbiological point of view, the reconstituted solution should be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and should not be longer than 24 hours including the duration of the treatment.
The other ingredients are: carbon dioxide and water for injections.
What Hemosol B0 looks like and contents of the pack
Hemosol B0 is presented in a two-compartment bag. The bag is over wrapped with a transparent film. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions.
The reconstituted solution is clear and colourless. Each bag (A+B) contains 5000 ml solution for haemofiltration, haemodiafiltration and/ or haemodialysis.
Each box contains two bags and one package leaflet.
Marketing Authorisation Holder
Gambro Lundia AB Magistratsvagen 16 SE-226 43 Lund Sweden
I 3 I
Hemosol B0 D05787001 Rev. 2015-02/1 REG 4741
Hemosol B0 solution for haemodialysis/haemofiltration
Carefully follow the instructions for use/ handling.
Solution A must be mixed with solution B before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
If heating of the solution to body temperature (+37°C) is necessary the procedure must be carefully controlled verifying that the solution is clear and without particles. Additional sodium bicarbonate substitution may increase the risk of metabolic alkalosis.
Before and during treatment, haemodynamic status, fluid balance, electrolyte and acid-base balance should be closely monitored. As Hemosol B0 is potassium-free, special attention should be given to potassium levels. Phosphate and potassium supplement might be necessary.
The volume of Hemosol B0 used will depend on the patient's clinical condition and the target fluid balance. Continued application of haemofiltration will remove excess fluid and electrolytes.
I 8 |
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored:
• In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced.
• In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
Overdose will result in fluid overload if the patient is suffering from renal failure, and it could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
The use of contaminated haemofiltration solution may cause sepsis and shock.
Instructions for use / Handling
Do not use with a haemodialysis monitor. You should only use monitors for Continuous Renal Replacement Therapies.
The solution is packaged in a two compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Hemosol B0 D05787001 Rev. 2015-02/1 REG 4741
Use only if the solution is clear and the over wrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution.
It is the responsibility of the physician to judge the compatibility of an additive medication with Hemosol B0 by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water within the pH limits of Hemosol B0 (pH limits of reconstituted solution is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
X...............................................................................................................................................................................................................................................................................................................................X
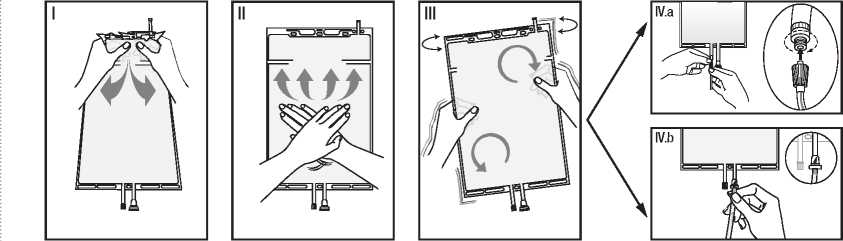
I Remove the over wrap from the bag immediately before use and discard any other packaging materials. Open the seal by holding the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Secure complete mixing of the solution by shaking the bag gently. The solution is now ready for use and the bag can be hung on the equipment.
(See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure IV.a below)
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below).
The solution should be used immediately after removal of the over wrap and after addition of solution A to solution B. If not used immediately, the reconstituted solution should be used within 24 hours, including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
o
Hemosol B0 D05787001 Rev. 2015-02/1 REG 4741
I 9 I
D05787001 Hemosol B0 PL w valve Non-PVC UK_IE_MT_PL_SI_2015-02_1 REG.indd 9 3/2/2015 6:07:28 PM