Hexvix 85 Mg Powder And Solvent For Solution For Intravesical Use


#
328
1045774
§ IPSEN
HEXVIX®
85 mg
powder and solvent for intravesical solution
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
HEXVIX® 85 mg, powder and solvent for intravesical solution
Hexaminolevulinate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor or nurse.
• If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Hexvix is and what it is used for
2. What you need to know before you are given Hexvix
3. How to use Hexvix
4. Possible side effects
5. How to store Hexvix
6. Contents of the pack and other information
1. What Hexvix is and what it is used for
This medicine is for diagnostic use only.
This medicine is used to help identify bladder cancers. It is given before your doctor uses a special device called a ‘cystoscope’ to look inside your bladder. A cystoscope helps to see possible tumours and thereby removal of abnormal cells, which illuminate in blue light after the administration of Hexvix.
2. What you need to know before you are given Hexvix
Do not have Hexvix
■ If you are allergic (hypersensitive) to the active ingredient or any other ingredients of Hexvix, including the liquid used to dissolve it (see section 6 'Contents of the pack and other information’).
• If you have ‘porphyria’ (a rare inherited blood disease).
Warnings and precautions
Check with your doctor before having Hexvix:
• If you have a urinary infection or burning feeling when you pass urine.
• If you have had BCG therapy on your bladder recently.
• If you have had an operation on your bladder recently.
These conditions may cause local reactions in your bladder, which can make it more difficult for your doctor to interpret what he sees during the examination.
Taking other medicines
Please tell your doctor if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may become pregnant or are planning to have a baby, ask your doctor for advice before having this medicine.
Driving and using machines
Talk to your doctor for advice about driving or using machines after having Hexvix.
3. How to use Hexvix
Hexvix will be prepared and given to you by a specially trained and qualified person.
• Hexvix is usually given in a hospital or clinic.
Your doctor will give it to you in the way described below:
1. A small tube called a 'catheter' will be placed into your bladder.
2. Your bladder will be emptied through this tube.
3. Hexvix will be put into your bladder through the tube.
4. Hexvix will be kept inside your bladder for about 60 minutes.
5. Your bladder will then be emptied through the tube.
6. The doctor will use a device (cystoscope) to look inside your bladder.
If you are given more Hexvix than you should
If Hexvix is kept in your bladder for more than 60 minutes or more Hexvix than usual is used, no side effects are expected. If you are concerned about this, speak to your doctor or nurse.
4. Possible side effects
Like all medicines, Hexvix can cause side effects, although not everybody gets them. There is a risk of side effects related to the examination technique (cystoscopy) used to look inside your bladder.
The use of Hexvix as a supportive procedure to standard cystoscopy for a more accurate diagnosis of your bladder cancer is in general well tolerated. If side effects happen, they are those typically associated with the standard examination technique, they are not usually serious and do not last very long. The following side effects may happen after the examination procedure using this medicine:
Common (affects 1 to 10 users in 100):
• Headache
• Feeling sick (nausea), vomiting
• Diarrhoea
• Constipation
■ Muscle cramp or pain in and around your stomach area (abdomen)
• Pain and difficulty passing urine
• Feeling unable to empty your bladder fully (urinary retention)
• Blood in your urine
• Pain after the examination (procedure)
• Fever (high temperature).
Uncommon (affects 1 to 10 users in 1,000):
■ Burning feeling when you pass urine (caused by inflammation or infection of your bladder)
• Needing to pass urine more often
■ Blood poisoning (septicaemia)
• Not being able to sleep or difficulty going to sleep
• Pain in the tube called the ‘urethra’ that urine passes through
• Feeling like you need to pass urine right away (urgency)
• Higher levels of white blood cells, increased levels of bilirubin (this is the yellowish pigment in your bile) or increased liver enzymes, these would all be seen in blood test results
■ Lower levels of red blood cells (anaemia)
• Inflammation of the head of the penis (balanitis)
• Back pain
• Gout
• Rash.
Frequency not known:
• Anaphylactoid shock (blood pressure drop, increased heart rate, skin rash).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system, listed below:
For Ireland: via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie: e-mail: medsafetv®hpra.ie
For UK: via the Yellow Card Scheme at: www.mhra.qov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Hexvix
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton. The expiry date refers to the last day of that month.
Powder and solvent: The product does not require any special storage conditions.
Solution (after mixing): Store at 2°C - 8°C (in a refrigerator) for not longer than 2 hours.
Hospital staff will ensure that the product is stored and disposed of correctly and not used after the expiry date stated on the outer carton.
6. Contents of the pack and other information
What Hexvix contains
• The active substance is hexaminolevulinate hydrochloride.
• The other ingredients are disodium phosphate dihydrate, potassium dihydrogen phosphate, sodium chloride, hydrochloric acid, sodium hydroxide and water for injections.
What Hexvix looks like and contents of the pack
• Each pack consists of a vial of white to off-white or pale yellow powder containing 85 mg of the active substance, hexaminolevulinate, and a polypropylene vial or prefilled syringe, containing 50 ml clear, colourless liquid to dissolve the powder.
• Hexvix powder is dissolved in the 50 ml solution provided in the pack. Once the powder and the solvent are mixed, a solution is made containing 1.7 mg/ml hexaminolevulinate, which corresponds to a 8 mmol/l solution of hexaminolevulinate.
Marketing Authorisation Holder
UK:
Ipsen Limited, 190 Bath Road, Slough, Berkshire, SL1 3XE, UK.
Ireland:
Ipsen Pharmaceuticals Limited, Blanchardstown Industrial Park, Blanchardstown, Dublin 15, Ireland.
Manufacturer
Photocure ASA Hoffsveien 4 NO-0275 Oslo Norway
For any information about this medicinal product, please contact the local representative of the Marketing Authorisation Holder.
UK:
Ipsen Limited 190 Bath Road Slough SL1 3XE United Kingdom.
Tel:+44 (0)1753 627777

0

Ireland:
Ipsen Pharmaceuticals Limited
Blanchardstown Industrial Park
Blanchardstown
Dublin 15
Ireland.
Tel: +353 (0) 1809 8256
This medicinal product is authorised under the trade name Hexvix in the following member states of the EEA: Austria, Belgium, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Iceland,
Italy, Latvia, Lithuania, Luxembourg, Malta, The Netherlands, Norway, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden and United Kingdom.
UK: This leaflet was last revised in February 2016.
Ireland: This leaflet was last revised in February 2016.
Licensed from: Photocure ASA.
Hexvix is a trademark of Photocure ASA.
<------------------------------------------------(tear off)
The following information is intended for healthcare professionals only:
Instructions for handling
Hexaminolevulinate may cause sensitisation by skin contact.
All steps should be performed with sterile equipment and under aseptic conditions.
Reconstitution procedure A: Hexvix Powder and Solvent for Hexvix in polypropylene vial
1. Withdraw 50.0 ml of the solvent for Hexvix into a sterile 50 ml syringe.
2. Inject about 10 ml of this solvent into the vial of Hexvix powder.
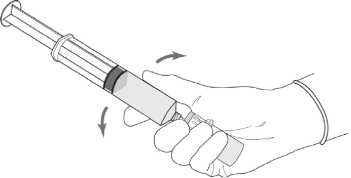
3. Without withdrawing the needle from the vial, hold the powder vial and the syringe in a firm grip and shake gently to ensure complete dissolution.
4. Withdraw all of the dissolved solution from the powder vial into the syringe. Gently mix the contents of the syringe.
5. Hexvix is now reconstituted and ready for use. The appearance of the reconstituted solution is clear to slightly opalescent, and colourless to pale yellow.
Reconstitution procedure B: Hexvix Powder and Solvent for Hexvix in prefilled syringe


6. Disconnect the empty vial from the syringe. Disconnect the needle from the syringe tip and discard it. Plug the syringe with the syringe cap. Gently mix the contents of the syringe. "
Hexvix is now reconstituted and ready for use. The appearance of the reconstituted solution is clear to slightly opalescent, and colourless to pale yellow.
Add two hours to the present time and write the resulting expiration time and date on the syringe label.
The product is for single use only. Any unused product should be discarded. No special requirements for disposal.
Chemical and physical stability of the solution has been demonstrated for 2 hours at 2°C - 8°C. From a microbiological point of view, the product should be used immediately. If not used immediately, inuse storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 2 hours at 2°C - 8°C.
0
1. Fasten the plunger rod into the rubber stopper of the syringe by turning the plunger rod clockwise until it stops.
2. Remove the cap from the syringe and keep it for later use. Connect a needle suitable for reconstitution to the syringe. Hold the syringe upright and carefully press the plunger rod upward to remove air.

4. Without withdrawing the needle from the vial, hold the powder vial and the syringe in a firm grip and shake gently to ensure complete dissolution.
328
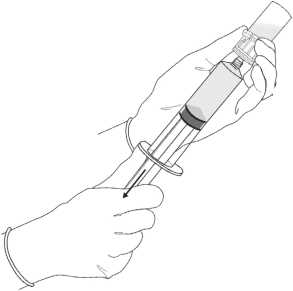
5. Withdraw all of the dissolved solution from the powder vial into the syringe.


PHARMACEUTICALDESIGN
|
1.4 |
s IP5EN |
DESIGN COLORS |
FONTS | |||
|
FRONT PAGE |
BACK PAGE |
Helvetica Neue 8 pt | ||||
|
Customer: |
Ipsen Pharma Biotech HexvixPFS 85 mg, leaflet GB |
Black |
Black | |||
|
Product | ||||||
|
Type |
Leaflet | |||||
|
Size: |
160 x 420 mm (Folded size: 80x52,5 mm) |
TECHNICAL COLORS | ||||
|
Requested by: |
Margaux Hamon |
1045774 | ||||
|
Requested date: |
04- 11 -2016 |
P pinkll | ||||
|
Artwork operator |
Rafael Cruz | |||||
|
Handling date: First version date |
04- 11 -2016 06-10 - 2016 | |||||
PHARMACEUTICALDESIGN, B.V.
Mauritskade 55c 1092 AD - Amsterdam +31(0)20 37047 26 info@pharmaceuticaldesign.com
# Boulogne-85_mg-Hexvix_PFS-UK-Leaflet-Ireland-(1.4).indd 3 4/11/16 12:14