Hrf 100mcg (Powder For Reconstitution For Injection)
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Gonadorelin 100 micrograms powder for solution for injection HRF 100 microgram
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 100 micrograms of gonadorelin as gonadorelin hydrochloride.
After reconstitution with 1 ml of water for injections, the resulting solution contains 100 micrograms/ml of gonadorelin as gonadorelin hydrochloride.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for solution for injection.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Gonadorelin as a single injection is indicated for evaluating the functional capacity and response of the gonadotropes of the anterior pituitary. The LH response is used in testing patients with suspected gonadotropin deficiency, whether due to the hypothalamus alone or in combination with anterior pituitary failure. Gonadorelin injection is also indicated for evaluating residual gonadotropic function of the pituitary following removal of a pituitary tumour or surgery and/or irradiation.
The gonadorelin test complements the clinical assessment of patients with a variety of endocrine disorders involving the hypothalamic-pituitary axis. In cases where there is a normal response, it indicates the presence of functional pituitary gonadotropes. The single injection test does not determine the patho-physiological cause for the subnormal response and does not measure pituitary gonadotropic reserve.
4.2 Posology and method of administration
Posology
Adults and Elderly
100 micrograms, subcutaneously or intravenously. In females for whom the phase of the menstrual cycle can be established, the test should be performed in the early follicular phase (days 1-7).
Paediatric population
The safety and efficacy of gonadorelin in children under one year have not been established. No data are available.
Route of administration
For subcutaneous and intravenous administration.
For instructions on reconstitution before administration, see section 6.6.
Test Methodology
To determine the status of the gonadotropin secretory capacity of the anterior pituitary, a test procedure requiring seven venous blood samples for LH is recommended.
Procedure:
1. Venous blood samples should be drawn at -15 minutes and immediately prior to gonadorelin administration. The LH baseline is obtained by averaging the LH values of the two samples.
2. Administer a bolus of gonadorelin subcutaneously or intravenously.
3. Draw venous blood samples at 15, 30, 45, 60 and 120 minutes after administration.
4. Blood samples should be handled as recommended by the laboratory that will determine the LH content. It must be emphasised that the reliability of the test is directly related to the inter-assay and intra-assay reliability of the laboratory performing the assay.
Interpretation of test results: Interpretation of the LH response requires an understanding of the hypothalamic-pituitary physiology, knowledge of the clinical status of the individual patient, and familiarity with the normal ranges and the standards used in the laboratory performing the LH assays.
LHiJmlU/ml)
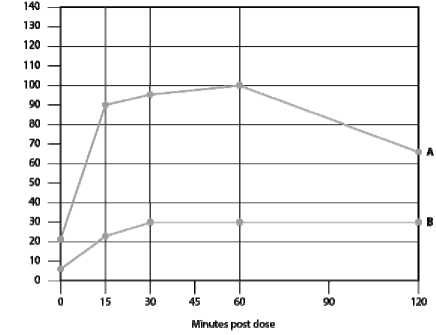
Figure 1. Normal male LH response after gonadorelin 100 micrograms subcutaneous administration 10th and 90th percentiles.
|
140 — 130 - |
, | |||
|
no — | ||||
|
90 — | ||||
|
! ™- | ||||
|
so - | ||||
|
30 -20 -10 - | ||||
|
1 |
1 |
a 15 SO 45 60 90 120
Minutes post dose
Figure 2. Normal male LH response after gonadorelin 100 micrograms intravenous administration 10th and 90th percentiles.
140 130 — 120 110 — IDO 90 — 60 70 —
20 j-10 —I
45
60
I
90
30 —
no —
so —
to —
50 —
30 —
Minutes post dose
Minutes post dose
Figure 3. Normal female LH response after gonadorelin 100 micrograms subcutaneous administration 10th and 90th percentiles.
Figure 4. Normal female LH response after gonadorelin
100 micrograms intravenous administration 10th and
90th percentiles.
Figures 1 - 4 represent the LH response curves after gonadorelin administration in normal subjects. The normal LH response curves were established between the 10th percentile (B line) and 90th percentile (A line) of all LH responses in normal subjects analysed from the results of clinical studies.
Individual patient responses should be plotted on the appropriate curve. A subnormal response in patients is defined as three or more LH values which fall below the B line of the normal LH response curve.
In cases where there is a blunted or borderline response, the gonadorelin test should be repeated.
The gonadorellin test complements the clinical assessment of patients with a variety of endocrine disorders involving the hypothalamic-pituitary axis. In cases where there is a normal response, it indicates the presence of functional pituitary gonadotropes. The single injection test does not determine the patho-physiological cause for the subnormal response and does not measure pituitary gonadotropic reserve.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Known or suspected pregnancy.
4.4 Special warnings and precautions for use
Although allergic and hypersensitivity reactions have been observed with other polypeptide hormones, to date no such reactions have been encountered following the administration of a single 100 micrograms dose of gonadorelin used for diagnostic purposes. Rare instances of hypersensitivity reactions have been reported. Therefore, patients in whom re-administration is considered, particularly by the intravenous route, should be carefully observed.
Administration during the follicular phase of a normal cycle may result in premature ovulation and appropriate measures are advised to prevent an unwanted pregnancy in these circumstances.
Gonadorelin and its analogues are not recommended for use in patients with pituitary adenoma as haemorrhagic infarction (pituitary apoplexy) may occur.
4.5 Interaction with other medicinal products and other forms of interaction
The gonadorelin test should be conducted in the absence of other drugs which directly affect the pituitary secretion of gonadotropins. These would include a variety of preparations which contain androgens, oestrogens, progestogens or glucocorticoids. The gonadotropin levels may be transiently elevated by spironolactone, minimally elevated by methyldopa, and suppressed by oral contraceptives and digoxin. The response to gonadorelin may be blunted by phenothiazines and dopamine antagonists which cause a rise in prolactin.
4.6 Pregnancy and lactation
Gonadorelin should not be administered to pregnant women or nursing mothers.
4.7 Effects on ability to drive and use machines
Gonadorelin has no or negligible influence on the ability to drive and operate machines.
4.8 Undesirable effects
The following adverse drug reactions have been identified or suspected in clinical trials and through post-marketing use. Adverse reactions listed below are classified according to frequency and System Organ Class (SOC).
Frequency categories are defined according to the following convention:
Very common (> 1/10), common (> 1/100 to < 1/10), uncommon (> 1/1,000 to < 1/100), rare (> 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data).
Cardiac disorders:
Rare: tachycardia
Gastrointestinal disorders:
Rare: nausea, abdominal discomfort
General disorders and administration site conditions:
Rare: induration at injection site
Immune system disorders:
Rare: hypersensitivity reactions
Infections and infestations:
Frequency unknown: sepsis
Investigations:
Rare: antibody test - positive for antibody formation
Nervous system disorders
Uncommon: pain Rare: headache, dizziness
Reproductive system and breast disorders:
Frequency unknown: menorrhagia
Respiratory, thoracic and mediastinal disorders:
Rare: bronchospasm
Skin and subcutaneous disorders:
Uncommon: swelling, pruritis
Rare: urticaria, erythema, erythema of eyelid
Frequency unknown: rash
Vascular disorders:
Rare: flushing
Frequency unknown: thrombophlebitis Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via www.mhra.gov .uk/yellowcard.
4.9 Overdose
Gonadorelin has been administered parenterally in doses up to 3 mg bd for 28 days without any signs or symptoms of overdosage. In cases of overdosage or idiosyncrasy, symptomatic treatment should be administered as required.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Gonadorelin stimulates the synthesis of follicle stimulating hormone and luteinising hormone in the anterior lobe of the pituitary as well as their release.
5.2 Pharmacokinetic properties
Gonadorelin is rapidly hydrolysed in plasma and excreted in urine with a half life of about 4 minutes.
5.3 Preclinical safety data
Non-clinical safety data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Lactose monohydrate
6.2 Incompatibilities
Gonadorelin should not be mixed with any other substance.
6.3 Shelf life
Unopened: 48 months After reconstitution: 24 hours.
6.4 Special precautions for storage
Store below 25°C.
6.5 Nature and contents of container
Gonadorelin is supplied in a USP Type I clear glass vial with grey butyl rubber stopper and aluminium collar.
6.6 Special precautions for disposal
Preparation for single injection administration: Reconstitute 100 micrograms vial with 1.0 ml of sterile water for injections. Prepare solution immediately before use. After reconstitution, refrigerate and use within 1 day. Discard unused reconstituted solution.
7 MARKETING AUTHORISATION HOLDER
Intrapharm Laboratories Limited
The Courtyard Barns
Choke Lane
Cookham Dean
Maidenhead
Berkshire SL6 6PT
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 17509/0005
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
25/05/2001 / 25/06/2004
10 DATE OF REVISION OF THE TEXT
27/08/2014