Imigran Injection
Out of date information, search anotherS240 LEAFLET Imigran 20150321 How to remove the used Cartridge Pack
S240 LEAFLET Imigran 20150321 How to remove the used Cartridge Pack
9. Return the used cartridge syringe to the empty space in the Cartridge Pack straight away.
10. Pushing the Pen down into the Cartridge Pack as far as it will go, unscrew the Pen by twisting it anti-clockwise (about half a turn) until it comes away.

1.
2.
3.
4.



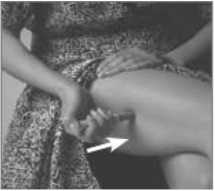
7. Press the Pen with the long blue Nose End firmly against a clean area of skin - usually the outside of the thigh (as in picture) - so the grey part slides down to cover part of the blue nose.
This releases the safety catch.
8. Hold the Pen firmly and press the
blue Release Button at the top of the Subject Pen. Count slowly to 10 keeping the Subject Pen very still and the Release Button depressed.
Do not take the Pen away from the skin too soon or some of the injection may be wasted.
Then lift the Pen away taking care not to touch the needle point.
11. Withdraw the Subject Pen from the Cartridge Pack. Close the blue hinged lid over the used syringe.
12. Put the Subject Pen back into the Carrycase and push it down until it stays down. It will click into place. The Subject Pen is then ready for use next time.
13. Close the Lid of the Carrycase until you need to use the next cartridge syringe. When you have used both cartridges, remove and replace the Cartridge Pack. (See right.)
When both syringes have been used, you can remove the Cartridge Pack.
Hold the Carrycase and press the two blue Locating Buttons with one hand.
Gently pull out the Cartridge Pack with the other hand.
Be careful to dispose of your empty Cartridge Packs safely. The Cartridge Pack will hold the used Subject cartridge syringes and needles until you can safely dispose of them.
You should be able to do this at your doctor’s surgery, so ask your doctor or practice nurse.
How to put a new Cartridge Pack into the Carrycase
Each Subject Pen comes complete with an Imigran Cartridge Pack
which contains two pre-filled cartridge syringes.
1. Swing open the Lid of the Carrycase, the Subject Pen is already in its place.
2. Push the Cartridge Pack into the Carrycase, pressing the blue buttons on either side so it slides in smoothly.
3. It does not matter which side of the Cartridge Pack is closest to the Subject Pen.
4. The Cartridge Pack is in the right position when the blue Locating Buttons show through the holes on either side of the Carrycase.
5. Close the Carrycase by swinging back the Lid and snapping it shut.
6. You can keep your Cartri+dge Pack safely in the Carrycase until you need to give yourself an injection.
7. Keep your Carrycase and any refill Cartridge Packs at a temperature below 30"C/86"F. If they are kept warmer than this for more than 24 hours it could spoil them.
S240 LEAFLET Imig-an 20150321
S240 LEAFLET Imigran 20150321
PACKAGE LEAFLET: INFORMATION FOR THE USER IMIGRAN® INJECTION (sumatriptan succinate)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed foryou only. Do not pass it onto others - it may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
See section 4.
What is in this leaflet
1 What Imigran Injection is and what it is used for
2 What you need to know before you use Imigran Injection
3 How to use Imigran Injection
4 Possible side effects
5 How to store Imigran Injection
6 Contents of the pack and other information
7 Step-by-step guide to using Imigran Injection
1 What Imigran Injection is and what it is used for
Each Imigran Injection pre-filled cartridge syringe contains a single dose of sumatriptan, which belongs to a group of medicines called triptans (also known as 5-HT1 receptor agonists).
Imigran Injection is used to treat migraine headache and a rare condition called cluster headache.
Migraine and cluster headache symptoms may be caused by the temporary widening of blood vessels in the head.
Imigran Injection is believed to reduce the widening of these blood vessels. This in turn helps to take away the headache and relieve other symptoms such as feeling or being sick (nausea or vomiting) and sensitivity to light and sound.
2 What you need to know before you use Imigran Injection
Don’t use Imigran Injection:
• If you’re allergic to sumatriptan, or any of the other ingredients of this medicine (listed in section 6)
• If you have a heart problem such as narrowing of the arteries (ischaemic heart disease) or chest pains (angina), or have already had a heart attack
• If you have circulation problems in your legs that cause cramp-like pains when you walk (peripheral vascular disease)
• If you have had a stroke or a mini-stroke (also called a transient ischaemic attack or TIA)
• If you have high blood pressure. You may be able to use Imigran if your high blood pressure is mild and is being treated
• If you have serious liver disease
• With other migraine medicines, including those which contain ergotamine, or similar medicines such as methysergide maleate; or any triptan or 5-HT! agonist (such as naratriptan or zolmitriptan)
• With any of the following anti-depressants:
• MAOIs (monoamine oxidase inhibitors) or if you have taken an MAOI in the last 2 weeks
• SSRIs (selective serotonin reuptake inhibitors) including citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline
• SNRIs (serotonin noradrenaline reuptake inhibitors) including venlafaxine orduloxetine.
• For children under 18 years of age.
If any of these apply to you:
Tell your doctor, and don’t use Imigran Injection.
Take special care with Imigran Injection
Talk to your doctor or pharmacist before using Imigran.
If you have any extra risk factors
• If you are a heavy smoker or are using nicotine replacement therapy, and especially
• If you are a man aged over 40, or
• If you are a woman who has been through the menopause
In very rare cases, people have developed serious heart conditions after using Imigran, even though they had no signs of heart disease before. If any of the points above applies to you it could mean you have a greater risk of developing heart disease - so:
Tell your doctor so that your heart function can be checked before Imigran is prescribed foryou.
If you have a history of fits (seizures)
Or if you have other conditions which might make it more likely that you’ll have a fit - for example, a head injury or alcoholism:
Tell your doctor so that you can be supervised more closely.
If you have had high blood pressure Imigran may not be suitable for you
Tell your doctor or pharmacist before using Imigran.
If you have liver or kidney disease If either of these apply to you:
Tell your doctor or pharmacist before using Imigran.
If you are allergic to antibiotics called sulphonamides
If so, you may also be allergic to Imigran. If you know you are allergic to an antibiotic but you are not sure whether it is a sulphonamide:
Tell your doctor or pharmacist before using Imigran.
If you are taking anti-depressants called SSRIs
(Selective Serotonin Reuptake Inhibitors) or SNRIs (Serotonin Noradrenaline Reuptake Inhibitors)
Tell your doctor or pharmacist before using Imigran.
Also see Other medicines and Imigran, below.
If you use Imigran frequently
Using Imigran too often may make your headaches worse.
Tell your doctor if this applies to you. He or she may
recommend you stop using Imigran.
If you feel pain or tightness in your chest after you use Imigran
These effects may be intense but they usually pass quickly. If they don’t pass quickly, or they become severe:
Get medical help immediately. Section 4 (below) has more information about these possible side effects.
Other medicines and Imigran
Tell your doctor or pharmacist if you’re taking, have recently taken or might take any other medicines. This includes any herbal products or medicines you’ve bought without a prescription.
Some medicines must not be taken with Imigran and others may cause adverse effects if they’re taken with Imigran.
You must tell your doctor if you are taking:
• ergotamine also used to treat migraine, or similar medicines such as methysergide (see section 2 Don’t use Imigran Injection). Don’t use Imigran at the same time as these medicines. Stop taking these medicines at least 24 hours before using Imigran. Don’t take any medicines which contain ergotamine or compounds similar to ergotamine again for at least 6 hours after using Imigran.
• other triptans/5-HTi receptor agonists (such as naratriptan, rizatriptan, zolmitriptan), also used to treat migraine, (see section 2 Don’t use Imigran Injection).
Don’t use Imigran at the same time as these medicines. Stop taking these medicines at least 24 hours before using Imigran. Don’t take anothertriptan/5-HT! receptor agonist for at least 24 hours after using Imigran.
• MAOIs used to treat depression. Don’t use Imigran if you have taken these in the last 2 weeks.
• SSRIs and SNRIs used to treat depression. Using Imigran with these medicines can cause serotonin syndrome (a collection of symptoms which can include restlessness, confusion, sweating, hallucinations, increased reflexes, muscle spasms, shivering, increased heartbeat and shaking). Tell your doctor immediately if you are affected in this way.
• St John’s Wort (Hypericum perforatum). Taking herbal remedies containing St John’s Wort while using Imigran may make side effects more likely.
Pregnancy and breast-feeding
• If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. There is only limited information about the safety of Imigran for pregnant women, though up till now there is no evidence of any increased risk of birth defects. Your doctor will discuss with you whether or not you should use Imigran while you are pregnant
• Don’t breast-feed your baby for 12 hours after using Imigran. If you express any breast milk during this time, discard the milk and don’t give it to your baby.
Driving and using machines
Either the symptoms of migraine or your medicine may make you
drowsy. If you are affected, don’t drive or operate machinery.
3 How to use Imigran Injection
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Imigran Injection is usually injected into the thigh.
There’s a step-by-step guide to using the syringe at the end of this leaflet (see section 7 overleaf).
When to use Imigran
• It’s best to use Imigran as soon as you feel a migraine or a cluster headache coming on, although you can use it at any time during an attack
• Don’t use Imigran to try to prevent an attack - only use it after your migraine symptoms start.
How much to use Adults aged 18 to 65
• The usual dose for adults aged 18 to 65 with migraine or a cluster headache is one 6 mg injection.
Children under 18
• Imigran is not recommended for children under 18 years old. Older people (aged over 65)
• Imigran is not recommended for people aged over 65.
If your symptoms start to come back
• You can use a second Imigran injection if at least 1 hour has passed since the first injection.
If the first injection has no effect
• Don’t use a new injection or any other Imigran preparation for the same attack.
If Imigran doesn’t give you any relief:
Ask your doctor or pharmacist for advice.
If you use more Imigran than you should
Using too much Imigran could make you ill. If you have used more than two injections in 24 hours:
Contact your doctor for advice.
If you have further questions about the use of this medicine, ask your doctor or pharmacist.
4 Possible side effects
Like all medicines, this medicine can cause side effects, but not everybody gets them. Some symptoms may be caused by the migraine itself.
Allergic reaction: get doctor’s help straight away
The following side effects have occurred but their exact frequency is not known.
• The signs of allergy include rash, hives (itchy rash); wheezing; swollen eyelids, face or lips; complete collapse.
If you get any of these symptoms soon after using Imigran:
Don’t use any more. Contact a doctor straight away.
Very common side effects
(affect more than 1 in 10 people)
• Temporary pain at the site of injection
• Stinging or burning, redness, swelling, bruising and bleeding at the site of injection.
Common side effects
(affect up to 1 in 10 people)
• Pain, heaviness, pressure or tightness in the chest, throat or other parts of the body, or unusual sensations, including numbness, tingling and warmth or cold. These effects may be intense but generally pass quickly.
If these effects continue or become severe (especially the chest pain):
Get medical help urgently. In a very small number of people these symptoms can be caused by a heart attack.
Other common side effects include:
• Feeling sick (nausea) or being sick (vomiting), although this may be due to the migraine itself
• Tiredness or drowsiness
• Dizziness, feeling weak, or getting hot flushes
• Temporary increase in blood pressure
• Shortness of breath
• Aching muscles.
Very rare side effects
(affect up to 1 in 10,000 people)
• Liver function changes. If you have a blood test to check your liver function, tell your doctor or nurse that you are using Imigran.
Some patients may have the following side effects but it is not known how often they occur
• Seizures/fits, tremors, muscle spasm, neck stiffness
• Visual disturbances such as flickering, reduced vision, double vision, loss of vision, and in some cases even permanent defects (although these may be due to the migraine attack itself)
• Heart problems, where your heartbeat may go faster, slower or change rhythm, chest pains (angina) or heart attack
• Pale, blue-tinged skin and/or pain in your fingers, toes, ears, nose or jaw in response to cold or stress (Raynaud’s phenomenon)
• Feeling faint (blood pressure may go down)
• Pain in the lower left side of the stomach and bloody diarrhoea (ischaemic colitis)
• Diarrhoea
• Pain in the joints
• Feeling anxious
• Excessive sweating.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
Release Button_
Subject Pen
Carrycase Nose End
White Rod (inside Nose End)
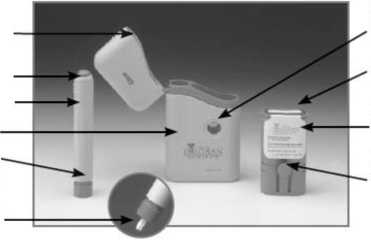
Seal
Cartridge
Pack
Locating
Buttons
. KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 30°C. Store in the original container to protect from light.
• Do not use after the expiry date printed on the pack label.
• Keep your injections in the case provided. You may wish to carry Imigran Injection with you in case of a migraine attack.
• Take the used injection pen and empty cartridges back to the pharmacist for disposal.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine fails to work correctly or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6 Contents of the pack and other information
What Imigran Injection contains
• Each 0.5ml pre-filled syringe contains 6mg of the active
ingredient sumatriptan (as the succinate) in a solution of sodium chloride and water for injection.
Cartridge
Button
Holes
How to give an injection using the Subject Pen
• Read the leaflet fully and carefully before using the Subject Pen
• Each Imigran Injection system comes complete with a Cartridge Pack which contains two cartridge syringes pre-filled with Imigran
• Use the photo on the left to help you identify the different parts of the injection system
• Do not load the Pen until you are ready to give the injection.
1
2
Swing open the Lid of the Carrycase.
Tear off the red Seal from one of the cartridges. Open the blue hinged lid underneath the Seal.

3 Take out the Subject Pen from the Carrycase. Check that the White Rod is not sticking out beyond the end of the Pen (see picturel 1). If it is sticking out, place the Pen back inside the Carrycase, push firmly and the Rod should click into place. The Pen is now ready for use.

What Imigran Injection looks like and contents of the pack
The carton contains a grey carrycase containing 1 Auto-injector pen (the injection device) and a blue Cartridge Pack containing two cartridge syringes pre-filled with the injection.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HAO 1DX.
Manufacturer
This product is manufactured by Glaxo Operations UK Ltd,
Barnard Castle, UK; and/or Vetter Pharma - Fertigung & Co. KG, Ravensburg, Germany; and/or GlaxoSmithKline Manufacturing
S.p.A., Strada Provinciale Asolana, n.90, San Polo di Torrile, Parma, Italy.
POM PL No: 19488/0240
Leaflet revision date: 21 March 2015
4 Push the Subject Pen firmly into the opened Cartridge Pack and gently screw it clockwise (about half a turn) until it will twist no further.


Imigran is a registered trade mark of Glaxo Group Ltd, UK.
7 Step-by-step guide to using your Imigran Injection
This leaflet shows you howto load the Subject Pen and howto use it to give a dose of Imigran medicine.
Please read this leaflet before using the injection system.
The GlaxoSmithKline Injection system is designed for use with a medicine called Imigran.
Each Subject injection system comes complete with an Imigran Cartridge Pack.
The Cartridge pack contains two pre-filled cartridge syringes. Subject refill packs containing one Imigran Injection Cartridge are also available.
Important: In the unlikely event that you have a problem with the Imigran Injection mechanism, please:
• Return it to your pharmacist who will replace it; or
• Contact GlaxoSmithKline Customer Contact Centre on Freephone 0800 221441 and they will tell you how to return it.
Keep out of the sight and reach of children
5 Keeping your finger away from the blue Release Button, pull the Subject Pen out of the Cartridge Pack. You may have to pull quite hard to do this. A safety catch stops accidental injection before you are ready.
6 The loaded Pen is now ready for immediate use.
Do not try to put the loaded Pen back into the Carrycase until after you have used the injection, orthe needle may be damaged and tine Pen will not inject correctly.
S240 LEAFLET lmig-an 20150321
9. Return the used cartridge syringe to the empty space in the Cartridge Pack straight away.
10. Pushing the Pen down into the Cartridge Pack as far as it will go, unscrew the Pen by twisting it anti-clockwise (about half a turn) until it comes away.

1.
2.
3.
4.



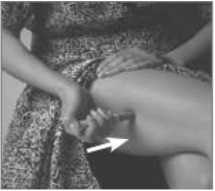
7. Press the Pen with the long blue Nose End firmly against a clean area of skin - usually the outside of the thigh (as in picture) - so the grey part slides down to cover part of the blue nose.
This releases the safety catch.
8. Hold the Pen firmly and press the
blue Release Button at the top of the Subject Pen. Count slowly to 10 keeping the Subject Pen very still and the Release Button depressed.
Do not take the Pen away from the skin too soon or some of the injection may be wasted.
Then lift the Pen away taking care not to touch the needle point.
11. Withdraw the Subject Pen from the Cartridge Pack. Close the blue hinged lid over the used syringe.
12. Put the Subject Pen back into the Carrycase and push it down until it stays down. It will click into place. The Subject Pen is then ready for use next time.
13. Close the Lid of the Carrycase until you need to use the next cartridge syringe. When you have used both cartridges, remove and replace the Cartridge Pack. (See right.)
When both syringes have been used, you can remove the Cartridge Pack.
Hold the Carrycase and press the two blue Locating Buttons with one hand.
Gently pull out the Cartridge Pack with the other hand.
Be careful to dispose of your empty Cartridge Packs safely. The Cartridge Pack will hold the used Subject cartridge syringes and needles until you can safely dispose of them.
You should be able to do this at your doctor’s surgery, so ask your doctor or practice nurse.
How to put a new Cartridge Pack into the Carrycase
Each Subject Pen comes complete with an Sumatriptan Cartridge
Pack which contains two pre-filled cartridge syringes.
1. Swing open the Lid of the Carrycase, the Subject Pen is already in its place.
2. Push the Cartridge Pack into the Carrycase, pressing the blue buttons on either side so it slides in smoothly.
3. It does not matter which side of the Cartridge Pack is closest to the Subject Pen.
4. The Cartridge Pack is in the right position when the blue Locating Buttons show through the holes on either side of the Carrycase.
5. Close the Carrycase by swinging back the Lid and snapping it shut.
6. You can keep your Cartridge Pack safely in the Carrycase until you need to give yourself an injection.
7. Keep your Carrycase and any refill Cartridge Packs at a temperature below 30"C/86"F. If they are kept warmer than this for more than 24 hours it could spoil them.
S240 LEAFLET Sumatriptan 20150321
S240 LEAFLET Sumatriptan 20150321
PACKAGE LEAFLET: INFORMATION FOR THE USER SUMATRIPTAN 6mg INJECTION (sumatriptan succinate)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed foryou only. Do not pass it onto others - it may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
See section 4.
What is in this leaflet
1 What Sumatriptan Injection is and what it is used for
2 What you need to know before you use Sumatriptan Injection
3 How to use Sumatriptan Injection
4 Possible side effects
5 How to store Sumatriptan Injection
6 Contents of the pack and other information
7 Step-by-step guide to using Sumatriptan Injection
1 What Sumatriptan Injection is and what it is used for
Each Sumatriptan Injection pre-filled cartridge syringe contains a single dose of sumatriptan, which belongs to a group of medicines called triptans (also known as 5-HT1 receptor agonists).
Sumatriptan Injection is used to treat migraine headache and a rare condition called cluster headache.
Migraine and cluster headache symptoms may be caused by the temporary widening of blood vessels in the head.
Sumatriptan Injection is believed to reduce the widening of these blood vessels. This in turn helps to take away the headache and relieve other symptoms such as feeling or being sick (nausea or vomiting) and sensitivity to light and sound.
2 What you need to know before you use Sumatriptan Injection
Don’t use Sumatriptan Injection:
• If you’re allergic to sumatriptan, or any of the other ingredients of this medicine (listed in section 6)
• If you have a heart problem such as narrowing of the arteries (ischaemic heart disease) or chest pains (angina), or have already had a heart attack
• If you have circulation problems in your legs that cause cramp-like pains when you walk (peripheral vascular disease)
• If you have had a stroke or a mini-stroke (also called a transient ischaemic attack or TIA)
• If you have high blood pressure. You may be able to use Sumatriptan if your high blood pressure is mild and is being treated
• If you have serious liver disease
• With other migraine medicines, including those which contain ergotamine, or similar medicines such as methysergide maleate; or any triptan or 5-HT! agonist (such as naratriptan or zolmitriptan)
• With any of the following anti-depressants:
• MAOIs (monoamine oxidase inhibitors) or if you have taken an MAOI in the last 2 weeks
• SSRIs (selective serotonin reuptake inhibitors) including citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline
• SNRIs (serotonin noradrenaline reuptake inhibitors) including venlafaxine orduloxetine.
• For children under 18 years of age.
If any of these apply to you:
Tell your doctor, and don’t use Sumatriptan Injection.
Take special care with Sumatriptan Injection
Talk to your doctor or pharmacist before using Sumatriptan.
If you have any extra risk factors
• If you are a heavy smoker or are using nicotine replacement therapy, and especially
• If you are a man aged over 40, or
• If you are a woman who has been through the menopause
In very rare cases, people have developed serious heart conditions after using Sumatriptan, even though they had no signs of heart disease before. If any of the points above applies to you it could mean you have a greater risk of developing heart disease - so:
Tell your doctor so that your heart function can be checked before Sumatriptan is prescribed foryou.
If you have a history of fits (seizures)
Or if you have other conditions which might make it more likely that you’ll have a fit - for example, a head injury or alcoholism:
Tell your doctor so that you can be supervised more closely.
If you have had high blood pressure Sumatriptan may not be suitable for you
Tell your doctor or pharmacist before using Sumatriptan.
If you have liver or kidney disease
If either of these apply to you:
Tell your doctor or pharmacist before using Sumatriptan.
If you are allergic to antibiotics called sulphonamides
If so, you may also be allergic to Sumatriptan. If you knowyou are allergic to an antibiotic but you are not sure whether it is a sulphonamide:
Tell your doctor or pharmacist before using Sumatriptan.
If you are taking anti-depressants called SSRIs
(Selective Serotonin Reuptake Inhibitors) or SNRIs (Serotonin Noradrenaline Reuptake Inhibitors)
Tell your doctor or pharmacist before using Sumatriptan.
Also see Other medicines and Sumatriptan, below.
If you use Sumatriptan frequently
Using Sumatriptan too often may make your headaches worse.
Tell your doctor if this applies to you. He or she may
recommend you stop using Sumatriptan.
If you feel pain or tightness in your chest after you use Sumatriptan
These effects may be intense but they usually pass quickly. If they don’t pass quickly, or they become severe:
Get medical help immediately. Section 4 (below) has more information about these possible side effects.
Other medicines and Sumatriptan
Tell your doctor or pharmacist if you’re taking, have recently taken or might take any other medicines. This includes any herbal products or medicines you’ve bought without a prescription.
Some medicines must not be taken with Sumatriptan and others may cause adverse effects if they’re taken with Sumatriptan.
You must tell your doctor if you are taking:
• ergotamine also used to treat migraine, or similar medicines such as methysergide (see section 2 Don’t use Sumatriptan Injection). Don’t use Sumatriptan at the same time as these medicines. Stop taking these medicines at least 24 hours before using Sumatriptan. Don’t take any medicines which contain ergotamine or compounds similar to ergotamine again for at least 6 hours after using Sumatriptan.
• other triptans/5-HTi receptor agonists (such as naratriptan, rizatriptan, zolmitriptan), also used to treat migraine, (see section 2 Don’t use Sumatriptan Injection).
Don’t use Sumatriptan at the same time as these medicines.
Stop taking these medicines at least 24 hours before using Sumatriptan. Don’t take anothertriptan/5-HT! receptor agonist for at least 24 hours after using Sumatriptan.
• MAOIs used to treat depression. Don’t use Sumatriptan if you have taken these in the last 2 weeks.
• SSRIs and SNRIs used to treat depression. Using Sumatriptan with these medicines can cause serotonin syndrome (a collection of symptoms which can include restlessness, confusion, sweating, hallucinations, increased reflexes, muscle spasms, shivering, increased heartbeat and shaking). Tell your doctor immediately if you are affected in this way.
• St John’s Wort (Hypericum perforatum). Taking herbal remedies containing St John’s Wort while using Sumatriptan may make side effects more likely.
Pregnancy and breast-feeding
• If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. There is only limited information about the safety of Sumatriptan for pregnant women, though up till now there is no evidence of any increased risk of birth defects. Your doctor will discuss with you whether or not you should use Sumatriptan while you are pregnant
• Don’t breast-feed your baby for 12 hours after using Sumatriptan. If you express any breast milk during this time, discard the milk and don’t give it to your baby.
Driving and using machines
Either the symptoms of migraine or your medicine may make you
drowsy. If you are affected, don’t drive or operate machinery.
3 How to use Sumatriptan Injection
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Sumatriptan Injection is usually injected into the thigh.
There’s a step-by-step guide to using the syringe at the end of this leaflet (see section 7 overleaf).
When to use Sumatriptan
• It’s best to use Sumatriptan as soon as you feel a migraine or a cluster headache coming on, although you can use it at any time during an attack
• Don’t use Sumatriptan to try to prevent an attack - only use it after your migraine symptoms start.
How much to use Adults aged 18 to 65
• The usual dose for adults aged 18 to 65 with migraine or a cluster headache is one 6 mg injection.
Children under 18
• Sumatriptan is not recommended for children under 18 years old.
Older people (aged over 65)
• Sumatriptan is not recommended for people aged over 65.
If your symptoms start to come back
• You can use a second Sumatriptan injection if at least 1 hour has passed since the first injection.
If the first injection has no effect
• Don’t use a new injection or any other Sumatriptan preparation for the same attack.
If Sumatriptan doesn’t give you any relief:
Ask your doctor or pharmacist for advice.
If you use more Sumatriptan than you should
Using too much Sumatriptan could make you ill. If you have used more than two injections in 24 hours:
Contact your doctor for advice.
If you have further questions about the use of this medicine, ask your doctor or pharmacist.
4 Possible side effects
Like all medicines, this medicine can cause side effects, but not everybody gets them. Some symptoms may be caused by the migraine itself.
Allergic reaction: get doctor’s help straight away
The following side effects have occurred but their exact frequency is not known.
• The signs of allergy include rash, hives (itchy rash); wheezing; swollen eyelids, face or lips; complete collapse.
If you get any of these symptoms soon after using Sumatriptan:
Don’t use any more. Contact a doctor straight away.
Very common side effects
(affect more than 1 in 10 people)
• Temporary pain at the site of injection
• Stinging or burning, redness, swelling, bruising and bleeding at the site of injection.
Common side effects
(affect up to 1 in 10 people)
• Pain, heaviness, pressure or tightness in the chest, throat or other parts of the body, or unusual sensations, including numbness, tingling and warmth or cold. These effects may be intense but generally pass quickly.
If these effects continue or become severe (especially the chest pain):
Get medical help urgently. In a very small number of people these symptoms can be caused by a heart attack.
Other common side effects include:
• Feeling sick (nausea) or being sick (vomiting), although this may be due to the migraine itself
• Tiredness or drowsiness
• Dizziness, feeling weak, or getting hot flushes
• Temporary increase in blood pressure
• Shortness of breath
• Aching muscles.
Very rare side effects
(affect up to 1 in 10,000 people)
• Liver function changes. If you have a blood test to check your liver function, tell your doctor or nurse that you are using Sumatriptan.
Some patients may have the following side effects but it is not known how often they occur
• Seizures/fits, tremors, muscle spasm, neck stiffness
• Visual disturbances such as flickering, reduced vision, double vision, loss of vision, and in some cases even permanent defects (although these may be due to the migraine attack itself)
• Heart problems, where your heartbeat may go faster, slower or change rhythm, chest pains (angina) or heart attack
• Pale, blue-tinged skin and/or pain in your fingers, toes, ears, nose or jaw in response to cold or stress (Raynaud’s phenomenon)
• Feeling faint (blood pressure may go down)
• Pain in the lower left side of the stomach and bloody diarrhoea (ischaemic colitis)
• Diarrhoea
• Pain in the joints
• Feeling anxious
• Excessive sweating.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
Release Button_
Subject Pen
Carrycase Nose End
White Rod (inside Nose End)
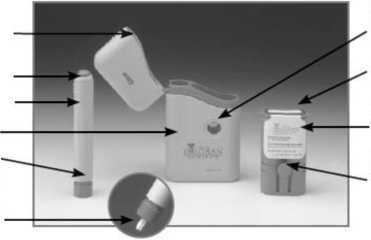
Seal
Cartridge
Pack
Locating
Buttons
Cartridge
Button
Holes
. KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 30°C. Store in the original container to protect from light.
• Do not use after the expiry date printed on the pack label.
• Keep your injections in the case provided. You may wish to carry Sumatriptan Injection with you in case of a migraine attack.
• Take the used injection pen and empty cartridges back to the pharmacist for disposal.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine fails to work correctly or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6 Contents of the pack and other information
What Sumatriptan Injection contains
• Each 0.5ml pre-filled syringe contains 6mg of the active
ingredient sumatriptan (as the succinate) in a solution of sodium chloride and water for injection.
How to give an injection using the Subject Pen
• Read the leaflet fully and carefully before using the Subject Pen
• Each Sumatriptan Injection system comes complete with a Cartridge Pack which contains two cartridge syringes pre-filled with Sumatriptan
• Use the photo on the left to help you identify the different parts of the injection system
1
2
What Sumatriptan Injection looks like and contents of the pack
The carton contains a grey carrycase containing 1 Auto-injector pen (the injection device) and a blue Cartridge Pack containing two cartridge syringes pre-filled with the injection.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HAO 1DX.
Do not load the Pen until you are ready to give the injection.
Swing open the Lid of the Carrycase.
Tear off the red Seal from one of the cartridges. Open the blue hinged lid underneath the Seal.

Manufacturer
This product is manufactured by Glaxo Operations UK Ltd,
Barnard Castle, UK; and/or Vetter Pharma - Fertigung & Co. KG, Ravensburg, Germany; and/or GlaxoSmithKline Manufacturing S.p.A., Strada Provinciale Asolana, n.90, San Polo di Torrile, Parma, Italy.
POM PL No: 19488/0240
3 Take out the Subject Pen from the Carrycase. Check that the White Rod is not sticking out beyond the end of the Pen (see picturel 1). If it is sticking out, place the Pen back inside the Carrycase, push firmly and the Rod should click into place. The Pen is now ready for use.

This leaflet shows you howto load the Subject Pen and howto use it to give a dose of Sumatriptan medicine.
Please read this leaflet before using the injection system.
The GlaxoSmithKline Injection system is designed for use with a medicine called Sumatriptan.
Each Subject injection system comes complete with an Sumatriptan Cartridge Pack.
The Cartridge pack contains two pre-filled cartridge syringes. Subject refill packs containing one Sumatriptan Injection Cartridge are also available.
Important: In the unlikely event that you have a problem with the Sumatriptan Injection mechanism, please:
• Return it to your pharmacist who will replace it; or
• Contact GlaxoSmithKline Customer Contact Centre on Freephone 0800 221441 and they will tell you how to return it.
Keep out of the sight and reach of children
5 Keeping your finger away from the blue Release Button, pull the Subject Pen out of the Cartridge Pack. You may have to pull quite hard to do this. A safety catch stops accidental injection before you are ready.
4 Push the Subject Pen firmly into the opened Cartridge Pack and gently screw it clockwise (about half a turn) until it will twist no further.


6 The loaded Pen is now ready for immediate use.
Do not try to put the loaded Pen back into the Carrycase until after you have used the injection, orthe needle may be damaged and the Pen will not inject correctly.
S240 LEAFLET Sumatriptan 20150321