Kolneb 1 Miu. Powder For Nebuliser Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Kolneb 1 MIU., Powder for Nebuliser Solution
Colistimethate sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Kolneb 1 MIU. is and what it is used for
2. What you need to know before you use Kolneb 1 MIU.
3. How to use Kolneb 1 MIU.
4. Possible side effects
5. How to store Kolneb 1 MIU.
6. Contents of the pack and other information
1. What Kolneb 1 MIU. is and what it is used for
Kolneb 1 MIU. is an antibiotic. It belongs to a group of antibiotics that are called polymyxins.
Kolneb 1 MIU. works by killing some types of bacteria that can cause various sorts of infections in people. Like all antibiotics, Kolneb 1 MIU. is only able to kill some types of bacteria so it is only suitable for treating some types of infection.
Kolneb 1 MIU. is an antibiotic powder for the treatment by inhalation of lung infections in patients with cystic fibrosis caused by susceptible Pseudomonas aeruginosa.
Kolneb 1 MIU. is breathed into the lungs (inhaled) using a nebuliser.
2. What you need to know before you use Kolneb 1 MIU.
Do not use Kolneb 1 MIU.
- If you are allergic (hypersensitive) to colistimethate sodium, colistin sulphate or to other polymyxins.
- If you suffer from myasthenia gravis (a rare disease where your muscles are extremely weak and get tired very quickly.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using
- If you have or have had kidney problems.
- If you suffer from porphyria (a rare metabolic disease that some people are born with).
- If you suffer from asthma.
If any of these apply to you, tell your doctor.
Other medicines and Kolneb 1 MIU.
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines, including medicines you bought without a prescription. These medicines may interfere with the effect of Kolneb 1 MIU.
• If you are taking antibiotics called aminoglycosides (which include gentamicin, tobramycin, amikacin and netilmicin) or cephalosporins, please tell your doctor. Taking Kolneb 1 MIU. at the same time as these other antibiotics could increase your risk of kidney problems.
■
• Kolneb 1 MIU. could prolong the effects of muscle relaxing medicines, which may be used as part of a general anaesthetic if you have an operation. If you need to have a general anaesthetic, tell the anaesthesist that you are taking Kolneb 1 MIU.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Kolneb 1 MIU. should only be given during pregnancy or breast-feeding when clearly needed.
Driving and using machines
Kolneb 1 MIU. may make you feel dizzy, confused or have problems with your sight, such as blurred vision. If this happens to you, do not drive or operate any tools or machines and talk to your doctor or pharmacist.
3. How to use Kolneb 1 MIU.
Kolneb 1 MIU. is for inhalation use.
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
|
Recommended Dose |
Maximum dose per day | |
|
Adults |
1 to 2 Million International Units (MIU) twice daily |
2 MIU three times a day |
|
Children (age 2 years to 11 years) and Adolescents (age 12 years to 17 years) |
1 to 2 Million International Units (MIU) twice daily |
2 MIU three times a day |
|
Children younger than 2 years |
0.5 to 1 Million International Units (MIU) twice daily |
2 MIU |
Tell your doctor if you have problems with your kidneys as you may need to take a lower dose of Kolneb 1 MIU.
Use in children
In premature and newborn infants special care should be employed as renal function is only insufficiently developed in this population.
Please note Kolneb 1 MIU. is also available as 2 MIU vial.
You should take your first dose of Kolneb 1 MIU. when you are with your doctor or nurse.
Take your Kolneb 1 MIU. after physiotherapy (if you have physiotherapy) and after taking any other nebulised medicines that you have been prescribed.
Duration of treatment
Your doctor will tell you how long your treatment with Kolneb 1 MIU. will last. Do not stop treatment early because when treating bacterial infections it is important to complete the full course of treatment to reduce the risk of resistance formation of the infectious bacteria.
Preparation for inhalation treatment
If you are treating yourself at home, your doctor or nurse will show you how to use Kolneb 1 MIU. in your nebuliser when you first start this treatment.
Before Kolneb 1 MIU. can be used it must be dissolved in isotonic saline solution (salt water).
To start your treatment, you will need the following:
• One clear-glass vial of Kolneb 1 MIU.
• The solvent for dissolving the powder (3 ml of isotonic saline solution)
• A nebuliser appropriate for inhalation use of Kolneb 1 MIU. (e.g. PARI LC PLUS, PARI LC SPRINT, or eFlow rapid)
It is important that your nebuliser system functions properly before starting your treatment with Kolneb 1 MIU. Read carefully the instructions for use of the nebuliser for further information on handling the nebuliser system.
Place the components of your nebuliser on a clean flat surface and follow the manufacturer’s instructions for use.
Preparing your Kolneb 1 MIU. for inhalation
Kolneb 1 MIU. must be used immediately after dissolution. Do not dissolve Kolneb 1 MIU. until ready to administer a dose (see also section 5).
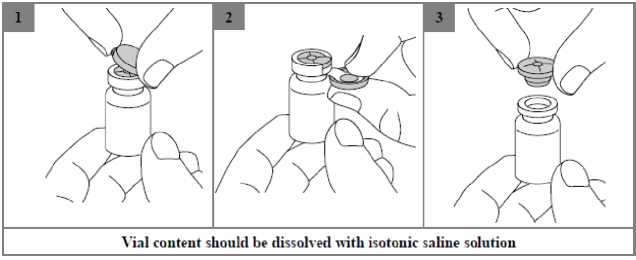
Step 1: Take one vial of Kolneb 1 MIU. and gently tap the glass vial so that the powder settles to the bottom. This helps ensure you get the proper dose of medication. Open the drug vial by lifting up the plastic overcap on the top (Figure 1).
Step 2: Pull down to carefully remove the entire plastic overcap together with metal ring from the vial (Figure 2). Safely dispose of the ring and overcap.
Step 3: Carefully remove the rubber stopper (Figure 3). Add the solvent (3 ml of isotonic saline solution) to the corresponding vial to dissolve the powder:
In order to avoid foaming, shake the vial gently until all powder is dissolved. Pour the solution into the nebuliser. Do not use Kolneb 1 MIU. if you notice visible particles in the solution after dissolution.
Once prepared Kolneb 1 MIU. should be used immediately. Any unused solution should be disposed of.
Using your Kolneb 1 MIU.
Kolneb 1 MIU. is for inhalation use with an appropriate nebuliser (e.g. PARI LC PLUS, PARI LC SPRINT or eFlow rapid).
For more detailed information on correct use of the selected nebuliser follow the instruction manual of the nebuliser.
Inhalation should take place in a well ventilated room.
After inhalation of Kolneb 1 MIU.
nebuliser for cleaning and disinfecting
Please refer to the manufacturer’s instructions for use of the instructions.
If you use more Kolneb 1 MIU. than you should
doctor has recommended (or if someone or pharmacist straight away.
If you realise that you have used more Kolneb 1 MIU. than your else has used some of your Kolneb 1 MIU.), contact your doctor The symptoms of taking too much Kolneb 1 MIU. can include:
• tingling or numbness around the lips and face
• dizziness and spinning sensation (vertigo)
• slurred speech
• visual disturbance
• confusion
• mental disturbance
• flushing (reddening of the face)
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
If you forget to use Kolneb 1 MIU.
Take the dose as soon as you remember, unless it is near the time for your next dose. You do not need to make up for the dose you have missed.
If you stop using Kolneb 1 MIU.
Do not stop your treatment early unless your doctor says that you can. Your doctor will tell you how long your treatment will last.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Kolneb 1 MIU. can sometimes cause allergic reactions like skin rash. If this happens you should stop taking Kolneb 1 MIU. and tell your doctor immediately.
Breathing in Kolneb 1 MIU. through a nebuliser can make some people notice tightness in their chest, feel wheezy, cough or become breathless. For this reason the first dose should be taken when you are with your doctor or nurse. Your doctor may also advise you to take a medicine to help prevent any breathlessness.
Your doctor may check your breathing at your clinic visits.
Kolneb 1 MIU. might also affect your kidneys, usually if the dose is high or you are taking other medicines that may affect your kidneys.
Kolneb 1 MIU. may sometimes cause you to have a sore mouth or sore throat.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Website: www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Kolneb 1 MIU.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the vial label after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C.
Keep the vials in the outer carton.
Kolneb 1 MIU. Powder for nebuliser solution should be used immediately after preparation. If this is not possible, a Kolneb 1 MIU. solution should not be stored above 25°C and not longer than 24 hours.
Any remaining solution should be discarded.
For single use only.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Kolneb 1 MIU. contains
- The active substance is colistimethate sodium (also known as colistin).
Each vial contains 1 million International Units (IU) of colistimethate sodium, which weighs about 80 milligrams (mg).
- There are no other ingredients.
What Kolneb 1 MIU. looks like and contents of the pack
Kolneb 1 MIU. is a powder for nebuliser solution after reconstitution with an adequate volume of solvent.
1 MIU/vial: White to off-white powder in a 10 ml colourless glass vial with a red cap.
Also available:
2 MIU/vial: White to off-white powder in a 10 ml colourless glass vial with a lilac cap.
The product is available in the following pack sizes:
• 1 vial
• 2 vials
• 10 vials
• 14 vials
• 28 vials
• 56 vials
Not all pack sizes may be marketed.
Marketing Authorisation Holder
YES Pharmaceutical Development Services GmbH Bahnstr. 42 - 46 61381 Friedrichsdorf Germany
Manufacturer
Forest Tosara Ltd.
Unit 146 Baldoyle Industrial Estate
Baldoyle, Dublin 13
Ireland
This leaflet was last revised in {03/2014}.
6