Lanoxin Injection
10000000120290
Package Leaflet: Information for Healthcare Professionals
Other glycosides, including metabolites of digoxin, can interfere with the assays that are available and one should always be wary of values which do not seem commensurate with the clinical state of the patient.
Please refer to the Summary of Product Characteristics for complete prescribing information
Digoxin 0.025 % w/v
Solution for Injection
Lanoxin is indicated in the management of chronic cardiac failure where the dominant problem is systolic dysfunction. Its therapeutic benefit is greatest in those patients with ventricular dilatation. Lanoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation.
Lanoxin is indicated in the management of certain supraventricular arrhythmias, particularly chronic atrial flutter and fibrillation.
The dose of Lanoxin for each patient has to be tailored individually according to age, lean body weight and renal function. Suggested doses are intended only as an initial guide.
The difference in bioavailability between injectable Lanoxin and oral formulations must be considered when changing from one dosage form to another. For example, if patients are switched from oral to the i.v. formulation the dosage should be reduced by approximately 33 %.
Adults and children over 10 years
Parenteral Loading:
Note: For use in patients who have not been given cardiac glycosides within the preceding two weeks
The loading of parenteral Lanoxin is 500 to 1000 micrograms (0.5 to 1.0 mg) depending on age, lean body weight and renal function.
The loading dose should be administered in divided doses with approximately half the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours, assessing clinical response before giving each additional dose. Each dose should be given by intravenous infusion (Dilution Instructions: See below for Instructions for use and handling) over 10 to 20 minutes. Maintenance Dose:
The maintenance dosage should be based upon the percentage of the peak body stores lost each day through elimination. The following formula has had wide clinical use:

Maintenance Dose Where:
Peak Body Stores % Daily Loss
C„ is creatinine clearance corrected to 70 kg body weight or 1.73 m2 body surface area. If only serum creatinine (Sa) concentrations are available, a Ccr (corrected to 70 kg body weight) may be estimated in men as
= Peak body stores x % daily loss
~Too
= Loading Dose
= 14 + Creatinine Clearance (Ccr)/5.
Ccr = (140-age)
Scr (in mg/IOOml)
NOTE: Where serum creatinine values are obtained in micromol/L these may be converted to mg/100 ml (mg %) as follows:
Scr(mg/100ml) = Scr (micromol/L) x 113.12
10,000
= Scr (micromol/L)
8!L4
Where 113.12 is the molecular weight of creatinine.
For women, this result should be multiplied by 0.85.
NOTE: These formulae cannot be used for creatinine clearance in children.
In practice, this will mean that most patients will be maintained on 0.125 to 0.25 mg digoxin daily; however in those who show increased sensitivity to the adverse effects of digoxin, a dosage of 62.5 microgram (0.0625 mg) daily or less may suffice. Conversely, some patients may require a higher dose.
Neonates, infants and children up to 10 years of age (if cardiac glycosides have not been given in the preceding two weeks):
In the newborn, particularly in the premature infant, renal clearance of digoxin is diminished and suitable dose reductions must be observed, over and above general dosage instructions.
Beyond the immediate newborn period, children generally require proportionally larger doses than adults on the basis of body weight or body surface area, as indicated in the schedule below. Children over 10 years of age require adult dosages in proportion to their body weight.
Parenteral Loading:
The parenteral loading dose in the above groups should be administered in accordance with the following schedule:
Preterm neonates < 1.5 kg 20 microgram/kg over 24 hours
Preterm neonates 1.5 kg to 2.5 kg 30 microgram/kg over 24 hours
Term neonates to 2 years 35 microgram/kg over 24 hours
2 to 5 years 35 microgram/kg over 24 hours
5 to 10 years 25 microgram/kg over 24 hours
The loading dose should be administered in divided doses with approximately half the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours, assessing clinical response before giving each additional dose. Each dose should be given by intravenous infusion (Dilution Instructions: See below for Instructions for use and handling) over 10 to 20 minutes.
Maintenance Dose:
The maintenance dose should be administered in accordance with the following schedule:
Preterm neonates:
daily dose = 20% of 24-hour loading dose (intravenous or oral) Term neonates and children up to 10 years:
daily dose = 25% of 24-hour loading dose (intravenous or oral)
These dosage schedules are meant as guidelines and careful clinical observation and monitoring of serum digoxin levels (see Monitoring) should be used as a basis for adjustment of dosage in these paediatric patient groups.
If cardiac glycosides have been given in the two weeks preceding commencement of Lanoxin therapy, it should be anticipated that optimum loading doses of Lanoxin will be less than those recommended above.
Use in the elderly:
The tendency to impaired renal function and low lean body mass in the elderly influences the pharmacokinetics of Lanoxin such that high serum digoxin levels and associated toxicity can occur quite readily, unless doses of Lanoxin lower than those in non-elderly patients are used. Serum digoxin levels should be checked regularly and hypokalaemia avoided.
Dose recommendations in renal disorder or with diuretic therapy:
The dosing recommendations should be reconsidered if patients are elderly or there are other reasons for the renal clearance of digoxin being reduced. A reduction in both initial and maintenance doses should be considered.
In patients receiving diuretics and an ACE inhibitor, or diuretics alone, the withdrawal of digoxin has been shown to result in clinical deterioration.
Monitoring:
Serum concentrations of digoxin may be expressed in conventional units of nanogram/ml (ng/ml) or SI Units of nanomol/L (nmol/L). To convert ng/ml to nmol/L, multiply ng/ml by 1.28.
The serum concentration of digoxin can be determined by radioimmunoassay. Blood should be taken 6 hours or more after the last dose of Lanoxin. Several post hoc analyses of heart failure patients in the Digitalis Investigation Group trial suggest that the optimal trough digoxin serum level may be 0.5 ng/mL (0.64 nanomol/L) to 1.0 ng/mL (1.28 nanomol/L).
Digoxin toxicity is more commonly associated with serum digoxin concentration greater than 2 ng/mL. However, toxicity may occur with lower digoxin serum concentrations. In deciding whether a patient's symptoms are due to digoxin, the patient's clinical state together with the serum potassium level and thyroid function are important factors.
X-
Lanoxin is contraindicated in:
• Intermittent complete heart block or second degree atrioventricular block, especially if there is a history of Stokes-Adams attacks.
• Arrhythmias caused by cardiac glycoside intoxication.
• Supraventricular arrhythmias associated with an accessory atrioventricular pathway, as in the Wolff-Parkinson-White syndrome unless the electrophysiological characteristics of the accessory pathway and any possible deleterious effect of digoxin on these characteristics have been evaluated. If an accessory pathway is known or suspected to be present and there is no history of previous supraventricular arrhythmias, Lanoxin is similarly contraindicated.
• Ventricular tachycardia or ventricular fibrillation.
• Hypertrophic obstructive cardiomyopathy, unless there is concomitant atrial fibrillation and heart failure, but even then caution should be exercised if digoxin is to be used.
• Patients known to be hypersensitive to digoxin or other digitalis glycosides, or to any component of the preparation.
Warnings and precautions for dosage and administration only, please refer to the Summary of Product Characteristics for complete prescribing information.
Serum levels of digoxin may be increased by concomitant administration of the following:
Alprazolam, amiodarone, flecainide, gentamicin, indometacin, itraconazole, prazosin, propafenone, quinidine, quinine, spironolactone, macrolide antibiotics (e.g. erythromycin and clarithromycin), tetracycline (and possibly other antibiotics), trimethoprim, propantheline, atorvastatin, cidosporin, epoprostenol (transient) and carvedilol.
Serum levels of digoxin may be reduced by concomitant administration of the following:
Adrenaline (epinephrine), antacids, kaolin-pectin, some bulk laxatives, colestyramine, acarbose, salbutamol, sulfasalazine, neomycin, rifampicin, some cytostatics, phenytoin, metodopramide, penicillamine and the herbal remedy St John's wort (Hypericum perforatum). Calcium channel blocking agents may either increase or cause no change in serum digoxin levels. Verapamil, felodipine and tiapamil increase serum digoxin levels.
Nifedipine and diltiazem may increase or have no effect on serum digoxin levels. Isradipine causes no change in serum digoxin levels. Angiotensin converting enzyme (ACE) inhibitors may also increase or cause no change in serum digoxin concentrations.
Milrinone does not alter steady-state serum digoxin levels.
Digoxin is a substrate of P-glycoprotein.Thus, inhibitors of P-glycoprotein may increase blood concentrations of digoxin by enhancing its absorption and/or by reducing its renal clearance (Seesection 5.2 of the Summary of Product Characteristics, Pharmacokinetic Propertied.
Rapid intravenous injection can cause vasoconstriction producing hypertension and/or reduced coronary flow. A slow injection rate is therefore important in hypertensive heart failure and acute myocardial infarction. Administering Lanoxin to a patient with thyroid disease requires care. Initial and maintenance doses of Lanoxin should be reduced when thyroid function is subnormal. In hyperthyroidism there is relative digoxin resistance and the dose may have to be increased. During the course of treatment of thyrotoxicosis, dosage should be reduced as the thyrotoxicosis comes under control.
For elective direct current cardioversion of a patient who is taking digoxin, the drug should be withheld for 24 hours before cardioversion is performed. In emergencies, such as cardiac arrest when attempting cardioversion, the lowest effective energy should be applied. Direct current cardioversion is inappropriate in the treatment of arrhythmias thought to be caused by cardiac glycosides.
Many beneficial effects of digoxin on arrhythmias result from a degree of atrioventricular conduction blockade. However, when incomplete atrioventricular block already exists, the effects of a rapid progression in the block should be anticipated. In complete heart block the idioventricular escape rhythm may be suppressed.
The administration of digoxin in the period immediately following myocardial infarction is not contra-indicated. However, the use of inotropic drugs in some patients in this setting may result in undesirable increases in myocardial oxygen demand and ischaemia, and some retrospective follow-up studies have suggested digoxin to be associated with an increased risk of death. However, the possibility of arrhythmias arising in patients who may be hypokalaemic after myocardial infarction and are likely to be cardiologically unstable must be borne in mind. The limitations imposed thereafter on direct current cardioversion must also be remembered.
Digoxin should not be used in constrictive pericarditis unless it is used to control the ventricular rate in atrial fibrillation or to improve systolic dysfunction.
The intramuscular route is painful and is associated with muscle necrosis. This route cannot be recommended.
Lanoxin Injection can be administered undiluted or diluted with a 4-fold or greater volume of diluent. The use of less than a 4-fold volume of diluent could lead to precipitation of digoxin.
Lanoxin Injection, 250 micrograms/ml when diluted in the ratio of 1 to 250 (i.e. One 2 ml ampoule containing 500 micrograms added to 500 ml of infusion solution) is known to be compatible with the following infusion solutions:
• Sodium Chloride Intravenous Infusion, BP, 0.9% w/v
• Sodium Chloride (0.18% w/v) and Glucose (4% w/v) Intravenous Infusion, BP
• Glucose Intravenous Infusion, BP, 5% w/v
Chemical in-use stability has been demonstrated for up to 96 hours at ambient temperature (20-25°C).
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution / dilution (etc) has taken place in controlled and validated aseptic conditions.
Any unused solution should be discarded.
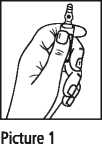
Ampoules
Ampoules are equipped with the OPC (One Point Cut) opening system and must be opened as follows:
• Hold with the hand the bottom part of the ampoule as indicated in Picture 1
• Put the other hand on the top of the ampoule positioning the thumb above the coloured point and press as indicated in Picture2

None known
Interactions may arise from effects on the renal excretion, tissue binding, plasma protein binding, distribution within the body, gut absorptive capacity and sensitivity to Lanoxin. Consideration of the possibility of an interaction whenever concomitant therapy is contemplated is the best precaution and a check on serum digoxin concentration is recommended when any doubt exists.
Digoxin, in association with beta-adrenoceptor blocking drugs, may increase atrio-ventricular conduction time.
Agents causing hypokalaemia or intracellular potassium deficiency may cause increased sensitivity to Digoxin; they include diuretics, lithium salts, corticosteroids and carbenoxolone.
Patients receiving Digoxin are more susceptible to the effects of suxamethonium-exacerbated hyperkalaemia.
Calcium, particularly if administered rapidly by the intravenous route, may produce serious arrhythmias in digitalized patients.
Do not store above 25°C Store in the original container
Aspen Pharma Trading Limited,
3016 Lake Drive,
Citywest Business Campus, Dublin 24, Ireland
PL 39699/0006
Leaflet date: December 2013
Lanoxin is a registered trademark of Aspen. All rights reserved.
aspen
aspen
Lanoxin® Injection
digoxin
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, nurse or pharmacist.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet please tell your doctor or pharmacist.
In this leaflet:
1 What Lanoxin is and what it is used for
2 Before you have Lanoxin
3 How to have Lanoxin
4 Possible side effects
5 How to store Lanoxin
6 Further information
Package Leaflet: Information for the User
Lanoxin contains a medicine called digoxin. This belongs to a group of medicines called 'cardiac glycosides'. They work by slowing down the rate while increasing the force of your heart when it beats.
It is used to treat certain heart problems, such as:
• heart failure
This is when your heart muscle can't pump strongly enough to supply blood around your whole body. It is not the same as a heart attack and does not mean that your heart stops.
• certain types of irregular heart beats
These include 'atrial flutter' or 'fibrillation'. They are caused by problems in the way the upper chambers of your heart send electrical signals. They cause your heart to beat too fast or in an uneven way.
Do not have Lanoxin if:
• you are allergic (hypersensitive) to digoxin, digitoxin or any of the other ingredients of Lanoxin (listed in Section 6)
• you have been told that you have any of the following heart problems:
- 'Second degree' or 'intermittent complete heart block'
- Certain types of 'supraventricular arrhythmias'
- 'Ventricular tachycardia' or 'Ventricular fibrillation'
- 'Hypertrophic obstructive cardiomyopathy'
Your doctor should have checked your heart problem and decided that this medicine will help you. If you are not sure, talk to your doctor, nurse or pharmacist before having Lanoxin.
Take special care with Lanoxin
Check with your doctor, nurse or pharmacist before having your medicine if:
• you have recently had a heart attack (myocardial infarction)
• you have been told that you have low potassium or magnesium levels in your blood (hypokalaemia or hypomagnesaemia)
• you have been told that you have high calcium levels in your blood (hypercalcaemia)
• you have a heart problem caused by a lack of vitamin B, known as 'Beri-Beri disease'
• you have kidney problems
• you have a lung problem - -
• you have thyroid problems
• you have digestion problems.
If you are not sure if any of the above apply to you, talk to your doctor, nurse or pharmacist before having this medicine. Your doctor may change your dose or you may need a different medicine.
Taking other medicines
Please tell your doctor, nurse or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription and herbal products.
In particular tell your doctor, nurse or pharmacist if:
• you have taken either digoxin or digitoxin in the last 2 weeks. Your doctor may need to change your dose.
Having Lanoxin with other medicines can change how they work or how Lanoxin works. Tell your doctor, nurse or pharmacist if you are taking any of the following:
• medicines for stomach problems, including indigestion, diarrhoea and being sick (vomiting)
• medicines for heart problems, including high blood pressure (hypertension) and irregular heart beat (arrhythmia)
• medicines for breathing problems, like asthma
• medicines for cancer
• medicines for epilepsy
• medicines for anxiety or depression
• medicines for bacterial infections (antibiotics)
• medicines for fungal infections (antifungals)
• medicines for high cholesterol
• medicines for preventing organ transplant rejection
• medicines for problems with your immune system
• medicines for preventing blood clots during kidney dialysis
• water tablets (diuretics)
• laxatives
• steroids
• anaesthetics
• the herbal remedy St John's wort (Hypericum perforatum). This should not be taken, when taking Lanoxin. If you already take St John's wort, speak to your doctor, as soon as possible, before you stop taking St John's wort.
If you are not sure if any of the above applies to you, talk to your doctor, nurse or pharmacist before taking Lanoxin.
Pregnancy and breast-feeding
Talk to your doctor before having this medicine if you are pregnant, might become pregnant or are breast-feeding.
Driving and using machines
You may feel dizzy, tired, have a headache or get blurred vision while having Lanoxin. If this happens, do not drive or use any tools or machines.
You will never be expected to give yourself this medicine. It will always be given to you by a person who is qualified to do so, like a nurse or a doctor. Your doctor will have decided how much Lanoxin is right for you:
• It depends on what heart problem you have and how serious it is.
• It also depends on your age, weight and how well your kidneys work.
• Your dose may go up or down depending on how you respond to the medicine. Your doctor will do checks to see how well the medicine is working. These may involve blood and urine tests.
Having this medicine
• Lanoxin will be given as a continuous infusion into your vein. This is where the drug is slowly given to you over a longer period of time, usually between 10 and 20 minutes.
• You usually have Lanoxin in two stages:
Stage 1 - loading dose
The loading dose gets your Lanoxin levels up to the correct level quickly.
Stage 2 - maintenance dose
After your loading dose you will take a much smaller
dose every day, until your doctor tells you to stop.
Adults and children over 10 years
• loading dose
- Usually between 0.5 and 1.0 mg (1 and 2 ampoules).
- This should be given in divided doses between 4 and 8 hours apart.
• maintenance dose
- Your doctor will decide this, depending on your response to Lanoxin.
- This will usually be given as either a tablet or an elixir.
Children under 10 years
• loading dose
- This is worked out using your child's weight
- Usually between 0.020 and 0.035 mg per kg of body weight.
- This should be given in divided doses between 4 and 8 hours apart.
• maintenance dose
- The doctor will decide this, depending on your child's response to Lanoxin.
- This will usually be given as either a tablet or an elixir.
If you have more Lanoxin than you should
If you have been given too much, tell your doctor or nurse
immediately. You may get any of the side effects and symptoms
listed in Section 4, but these can be serious.
If a dose of Lanoxin is missed
• If a dose is missed, tell your doctor or nurse and you may be
given the dose as soon as possible. However, if it is almost time
for the next dose, the missed dose will be skipped.
• Do not have a double dose to make up for the one that is missed.
If you have any further questions on having this medicine, ask your
doctor, nurse or pharmacist.
Uncommon (affects less than 1 in 100 people)
• depression.
Very Rare (affects less than 1 in 10,000 people)
• bruising or bleeding more easily than normal
• stomach pain caused by lack of blood supply or damage to your intestines
• mental disturbances, you may feel confused, indifferent or unable to judge clearly
• weakness, tiredness or a general feeling of being unwell
• breast enlargement in men
• loss of appetite
• headache.
Lanoxin can very rarely cause serious irregular heart rates. Your doctor may do regular checks to make sure Lanoxin is working safely for you.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, nurse or pharmacist.
• Keep out of the reach and sight of children.
• Do not use Lanoxin after the expiry date on carton or the ampoule label (Exp.). The expiry date refers to the last day of that month. It would have been checked by your doctor or nurse.
• Do not store above 25°C.
• Store in the original container.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Like all medicines, Lanoxin can cause side effects, although not everybody gets them. In general, the side effects tend to happen if the dose you are taking is too high, your doctor may adjust your dose.
Tell your doctor or nurse immediately if:
• you have palpitations, chest pain, shortness of breath or sweating. These can be symptoms of a serious heart problem caused by new irregular heart beats. If these happen, tell your doctor immediately.
Other side effects that you should tell your doctor about include:
Common (affects less than 1 in 10 people)
• slow or irregular heart rate
• feeling sick, being sick or diarrhoea
• skin rash that may be itchy
• drowsiness or dizziness
• visual disturbances, with blurred or yellow-green sight.
What Lanoxin contains
• The active ingredient is digoxin, each 2 ml contains 0.5 mg (500 micrograms).
• The other ingredients are ethanol, propylene glycol, citric acid, sodium phosphate and Water for Injections.
What Lanoxin looks like and contents of the pack
Each carton contains 5 clear glass ampoules of a clear and colourless solution.
Marketing Authorisation Holder and Manufacturer
Product licence held by Aspen Pharma Trading Limited,
3016 Lake Drive,
Citywest Business Campus, Dublin 24, Ireland Service-Tel:+800 00404142
Manufactured by GlaxoSmithKline Manufacturing S.p.A., San Polo di Torrile, Parma, Italy
PL 39699/0006
Leaflet date: December 2013
Lanoxin is a registered trademark of Aspen. All rights resented.
10000000120290
This PDF has been verified using PitStop 08 - PDF is PDF/X-1a compliant
(Illustrator files workflow)