Latanoprost 50 Micrograms/Ml Eye Drops Solution
Out of date information, search anotherPackage leaflet: Information for the user Latanoprost 50 micrograms/ml eye drops, solution
latanoprost
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your or your child’s doctor or pharmacist.
- This medicine has been prescribed for you or for your child. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your or your child’s doctoror pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What latanoprost eye drops are and what it is used for
2. What you need to know before you use latanoprost eye drops
3. How to use latanoprost eye drops
4. Possible side effects
5. How to store latanoprost eye drops
6. Contents of the pack and other information
Latanoprost belongs to a group of medicines known as prostaglandin analogues. It works by increasing the natural outflow of fluid from inside the eye into the bloodstream.
Latanoprost contains the active substance Latanoprost 0.005% w/v (50 micrograms per ml) eye drops solution.
Latanoprost is used to treat conditions known as open angle glaucoma and ocular hypertension. Both of these conditions are linked with an increase in the pressure within your eye, eventually affecting your eye sight.
Latanoprost is also used to treat increased eye pressure and glaucoma in all ages of children and babies.
Latanoprost can be used in adult men and women (including the elderly) and in children from birth to 18 years of age. Latanoprost has not been investigated in prematurely born infants (less than 36 weeks gestation).
Do not use Latanoprost eye drops
- if you are allergic (hypersensitive) to the active substance latanoprost or any of the other ingredients of Latanoprost eye drops (listed in section 6).
Warnings and precautions
Talk to your or your child’s doctor, pharmacist or nurse before using Latanoprost eye drops.
Tell your or your child’s doctor if you think any of the following apply to you or your child
• if you or your child have severe asthma, or the asthma is not well controlled.
• if you or your child had eye surgery such as cataract surgery or are about to have eye surgery.
• damage to the area around the lens of your eye.
• any medical condition that could affect your vision (such as diabetes).
• Swelling inside the eyeball (an eye condition known as macular oedema).
• if you or your child suffer from eye problems (such as eye pain, irritation or inflammation, blurred vision)
• if you or your child suffers from dry eyes
• if you have suffered or are currently suffering from a viral infection of the eye caused by the herpes simplex virus (HSV)
Other medicines and Latanoprost eye drops
Please tell your doctor or your child’s doctor if you or your child are taking or have recently taken or might take any other medicines, including other eye drops and any other medicines taken without a prescription. Latanoprost eye drops and other medicines taken at the same time can affect each other. Do not take Latanoprost eye drops with any other medicines containing prostaglandin because this may increase the pressure in your eye (s).
Pregnancy and breast-feeding
Do not use Latanoprost eye drops when you are pregnant. Tell your doctor immediately if you are pregnant, think you are pregnant, or are planning to become pregnant.
Do not use Latanoprost eye drops when you are breastfeeding.Ask your doctor or pharmacist foradvice before taking any medicine.
Children
In children from 0 to < 3 years old that mainly suffers from PCG (Primary Congenital Glaucoma), surgery (e.g. trabeculotomy/goniotomy) remains the first line treatment. Long-term safety in children has not yet been established.
No data are available for prematureinfants (less than 36 weeks gestational age).
Driving and using machines
When you use Latanoprost eye drops you might have blurred vision, for a short time. If this happens to you, do not drive or use any tools or machines until your vision becomes clear again.
Latanoprost eye drops contains a preservative called benzalkonium chloride
This preservative may cause eye irritation or disruption to the surface of the eye. Benzalkonium chloride can be absorbed by contact lenses and is known to discolour soft contact lenses. Therefore, avoid contact with soft contact lenses.
If you or your child wear contact lenses, they should be removed before using Latanoprost eye drops. After using Latanoprost eye drops you should wait 15 minutes before putting the contact lenses back in. See the instructions for contact lens wearers in Section 3.
Always use Latanoprost eye drops exactly as your or your child’s doctor has told you.
You should check with your or your child’s doctor or pharmacist if you are not sure.
The usual dose for children and adults, including elderly patients is One drop of Latanoprost eye drops should be dropped into the affected eye(s) once daily. The best time to do this is in the evening.
Do not use Latanoprost eye drops more than once a day, because the effectiveness of the treatment can be reduced if you administer it more often.
Use Latanoprost eye drops as instructed by your doctor or the doctor treating your child until they tell you to stop.
If you useLatanoprost eye drops with other eye drops:
Wait at least five minutes between using Latanoprost eye drops and taking other eye drops.
Contact lens wearers
If you or your child wear contact lenses, they should be removed before using Latanoprost eye drops. After using Latanoprost eye drops you should wait 15 minutes before putting the contact lenses back into the eyes.
Method of using Latanoprost eye drops
Follow the steps below to help you use Latanoprost eye drops properly:
1. Wash your hands and sit or stand comfortably.
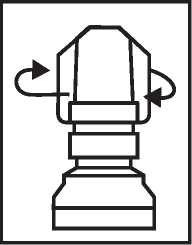
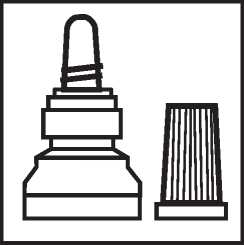
2. Twist off the outer cap (which can be thrown away)
3. Unscrew the protective inner cap.
The protective cap should be retained.
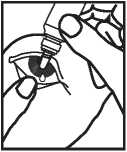
4. Use your finger to gently pull down the lower eyelid of your affected eye.
5. Place the tip of the bottle close to, but not touching your eye.
XXXXX
6. Squeeze the bottle gently so that only one drop goes into your eye and then release the lower eyelid.
7. Press a finger against the corner of the affected eye by the nose. Hold for 1 minute whilst keeping the eye closed.
8. Repeat in your other eye if your doctor has told you to do this.
9. Put the cap back on the bottle.
If you use more Latanoprost eye drops than you should
If you put too many drops in your eye, you may feel some slight irritation in the eye, have watery eyes or some redness around the eye. This should pass, but if you are worried contact your doctor orthe doctor treating your child for advice.
Contact your doctor as soon as possible if you or your child swallows Latanoprost eye drops accidentally If you forget to use Latanoprost eye drops
If you forget to apply your drops at the usual time, just skip that dose. Do not take a double dose to make up for the missed dose. Instead, just wait until the next time you are supposed to take it, and then take your normal dose.
If you stop using Latanoprost eye drops
Latanoprost eye drops should be used until your doctor tells you to stop. If you stop using this medicine the pressure in your eye could increase. You should speak to your doctor or the doctor treating your child if your child or you want to stop taking Latanoprost eye drops.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Latanoprost eye drops can cause side effects, although not everybody gets them.
The following are known side effects of using Latanoprost eye drops:
Very common: (may affect more than 1 in 10 people):
• A gradual change in your eye colour by increasing the amount of brown pigment in the coloured part of the eye known as the iris. If you have mixed-colour eyes (blue-brown, grey-brown, yellow-brown or green-brown) you are more likely to see this change than if you have eyes of one colour (blue, grey, green or brown eyes). Any changes in your eye colour may take years to develop although it is normally seen within 8 months of treatment. The colour change may be permanent and may be more noticeable if you use Latanoprost eye drops in only one eye. There appears to be no problems associated with the change in eye colour. The eye colour change does not continue after Latanoprost eye drops treatment is stopped
• Redness of the eye
• eye irritation (a feeling of burning, grittiness, itching, stinging or the sensation of a foreign body in the eye)
• A gradual change to eyelashes of the treated eye and the fine hairs around the treated eye, seen mostly in people of Japanese origin. These changes involve an increase of the colour (darkening), length, thickness and number of your eye lashes
Common: (may affect up to 1 in 10 people):
• irritationor disruption to the surface of the eye, eyelid inflammation (blepharitis)eye pain and light sensitivity (photophobia).
Uncommon: (may affect up to 1 in 100 people):
• eyelid swelling
• dryness of the eye
• inflammation or irritation of the surface of the eye (keratitis)
• blurred vision
• conjunctivitis
• skin rash
Rare: (may affect up to 1 in 1000 people):
• inflammation of the inner, middle or coloured part of your eye (iritis/uveitis)
• swelling of the retina (macular oedema)
• symptoms of swelling or scratching/damage to the surface of the eye
• swelling around the eye (periorbital oedema)
• eyelashes growing in the wrong direction sometimes resulting in eye irritation
• Skin reactions on the eyelids, darkening of eye lids or skin around the eye. These changes may be more noticeable if you are only treating one eye
• growth of an extra row of eyelashes
• asthma, worsening of asthma and difficulty in breathing Very rare: (may affect up to 1 in 10,000 people)
• worsening of angina (chest pain) in patients who also have heart disease
• chest pain
• sunken eye appearance (eye sulcus deepening)
There have also been reports of the following:
• fluid filled area within the coloured part of the eye (iris cyst)
• headache, dizziness
• awareness of your heartbeat (palpitations)
• muscle pain; joint pain
• developing a viral infection of the eye caused by the herpes simplex virus (HSV).
Side effects seen more often in children compared to adults are runny itchy nose and fever.
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Reporting of side effects
If you get any side effects, talk to your doctor,pharmacistor nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below).
By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom: Yellow Card Scheme at: www.mhra.gov.uk/yellowcard Ireland:
HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie E-mail: medsafety@hpra.ie
Malta: ADR Reporting: at: www.medicinesauthority.gov.mt/adrportal
Keep this medicine out of the sight and reach of children.
Do not use Latanoprost eye drops after the expiry date which is stated on the carton/label/box. The expiry date refers to the last day of that month.
Store the unopened bottles in a refrigerator (2- 8°C).
After first opening the bottle it is not necessary to store the bottle in a refrigerator but do not store above 25°C. Use within 4 weeks of opening. When you are not using this medicine, keep the bottle in outer carton, in order to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
What Latanoprost eye drops contain
• The active substance is 0.005%w/v (50 micrograms in one ml) latanoprost.
• The other ingredients are: sodium chloride, benzalkonium chloride, sodium dihydrogen phosphatemonohydrate (E339), anhydrous disodium phosphate (E339), water for injections
What Latanoprost eye drops look like and contents of the pack
Clear, colourless solution practically free from particles filled in 5 ml FFS vial. pH: 6.40-7.00
Osmolality: 240-300 mOsmols / kg
Polyethylene bottle with HDPE dropper inserts & white opaque HDPE cap & translucent LDPE tamper evident overcap.
Each dropper container contains 2.5 ml eye drops solution corresponding to approximately 80 drops of solution.
Pack sizes: 1 x 2.5 ml, 3 x 2.5 ml, 6 x 2.5 ml.
Not all pack sizes may be marketed.
Marketing Authorisation Holder andManufacturer Marketing Authorisation Holder
Cipla (EU) Limited
Hillbrow House,Hillbrow Road, Esher,Surrey, KT10 9NW, United Kingdom.
Manufacturer:
S&D Pharma CZ,
Cipla
spol. s r.o. Theodor 28, 273 08 Pchery (Pharmos a.s. facility), Czech republic. Cipla (EU) Limited
4th floor, 1 Kingdom Street, London, W2 6BY, United Kingdom.
This leaflet was last revised in 05/2015