Lifsar 50 Microgram/500 Microgram Per Metered Dose Inhalation Powder
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Lifsar 50 microgram/500 microgram per metered dose Inhalation powder
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each single inhalation provides a delivered dose (the dose leaving the mouthpiece) of 45 micrograms of salmeterol (as salmeterol xinafoate) and 465 micrograms of fluticasone propionate. This corresponds to a metered dose of 50 micrograms of salmeterol (as salmeterol xinafoate) and 500 micrograms of fluticasone propionate.
Excipient with known effect: contains up to 7 milligrams of lactose monohydrate per metered dose.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Inhalation powder.
The inhaler contains white powder. The inhaler body is grey and white with a grey base and mouthpiece, a white cover and a purple or grey bottom lid.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Lifsar is indicated for the symptomatic treatment of adults with Chronic Obstructive Pulmonary Disease (COPD) with a FEV1 < 60% predicted normal (pre-bronchodilator) and a history of repeated exacerbations and who have significant symptoms despite regular bronchodilator therapy.
Lifsar is intended for use by adults 18 years of age and older only.
4.2 Posology and method of administration
Posology
Route of administration; inhalation use.
Patients should be made aware that Lifsar must be used regularly, every day for optimum benefit, even when asymptomatic.
Lifsar is available in the strength of 50 micrograms of salmeterol and 500 micrograms of fluticasone propionate per metered dose only.
Recommended doses
Adults:
One inhalation of 50 micrograms salmeterol and 500 micrograms fluticasone propionate twice daily.
Special patient groups
There is no need to adjust the dose in elderly patients or in those with renal impairment.
There are no data available for use of Lifsar in patients with hepatic impairment.
Paediatric population
Lifsar is not for use in children and adolescents less than 18 years of age.
Method of administration
Patients should be instructed in the proper use of their inhaler (see patient information leaflet).
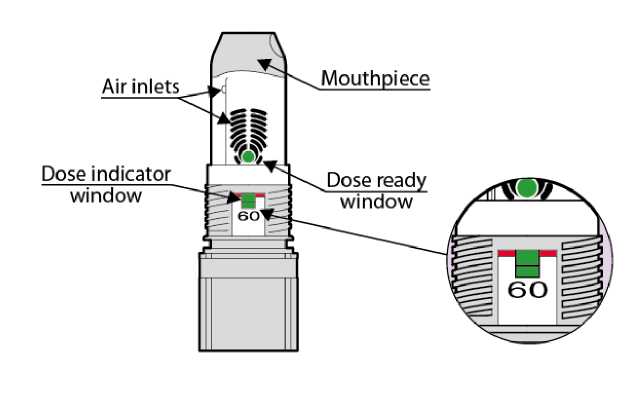
Note: The number shown at the dose indicator window refers to the initial number of inhalations (60) in the device and it doesn't change even if the device is empty. The green colour in the dose indicator window will show approximately how many inhalations are left (see below in text the section “When should the inhaler be replaced”).
The basic principles for using Lifsar are
1. OPEN: Remove the white cover.
2. INHALE: Place lips around the mouthpiece and inhale deeply.
3. CLOSE TO CLICK: Replace the cover fully.
Instructions for use
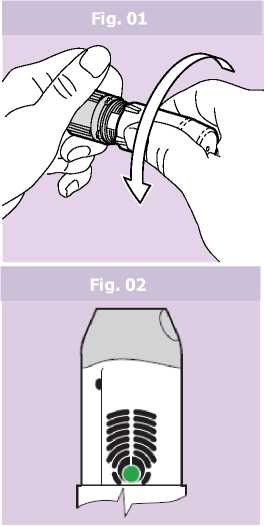
Fig. 03
1. OPEN
• Hold the device with two hands: one hand on the grey base and the other on the protective white cover. The device can be held in any position.
• The white protective cover should be removed from the grey base by twisting both parts in opposite directions. (Fig. 01). The patient will feel a small resistance when the cover is half opened.
• The green colour in the dose ready window confirms that the inhaler is ready for use (Fig. 02).
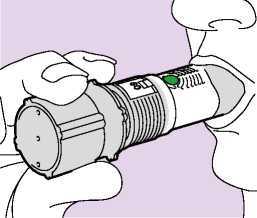
2. INHALE
• Hold the inhaler firmly by the grey base, away from the mouth. Breathe out slowly as far as is comfortable.
The patient should not breathe through the inhaler.
• The lips should be placed around the grey mouthpiece (Fig. 03) without covering any of the air inlets with the lips. The patient should not chew or bite the mouthpiece.
• The patient should breathe in as deeply and as hard as they can through their mouth (not through their nose).
• The patient should not stop inhaling when they hear a soft "plopping" sound. The soft “plopping” sound during the inhalation process indicates that the dose has been released.
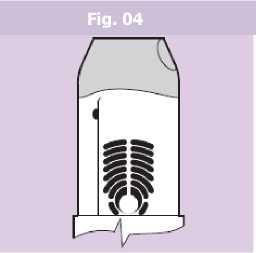
• The patient should hold their breath for an additional 5 - 10 seconds or as long as is comfortable before breathing out slowly through their nose and returning to normal breathing.
• The green colour in the dose ready window should now have disappeared, showing that the dose has been successfully delivered (Fig. 04).
3. CLOSE TO CLICK
Fig. 05
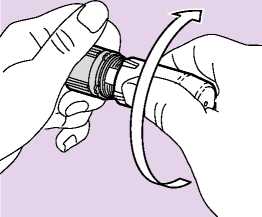
Fig. 06
• Twist the white protective cover back firmly onto the grey base until it clicks (Fig. 05).
• It is important that the cover is fully rotated until
it clicks as this loads the next dose of medicine. The alignment lines on the cover and base should match.
• If more than one inhalation is required, as instructed by the physician, the patient should repeat the above steps.
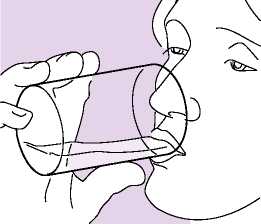
• The patients should be instructed to rinse their mouth with water (and spit it out) and/or to brush their teeth after each dose in order to minimise the risk of oropharyngeal candidiasis and hoarseness (Fig 06).
When should the inhaler be replaced?
Fig. 07
|
\m i | ||
|
IT 60 |
r1 | |
Full
|
m i | ||
|
P |
60 |
( |
|
1 J II |
1 Half full m i | |
|
( |
rs- 60 | |
T
Empty
|
JJ |
m i | |
|
i |
60 | |
10 remaining doses
• The number of initial inhalations (60) in the device is shown in the dose indicator window (Fig. 07). This number does not change even if the device is empty.
• The green colour in the dose indicator window shows approximately how many inhalations of the medicine are left in the device. Hold the inhaler upright at eye level to check how much is left in the container.
• When the green colour reaches the level of the red line, it means there are approximately 10 inhalations left in the device. (Fig. 07 - 10 remaining doses). The patient can continue using the inhaler, but should see their doctor for a new prescription.
• When the green indicator is no longer visible the inhaler is empty (Fig. 07 -Empty).
Cleaning the inhaler
• The mouthpiece can be cleaned by wiping it with a dry, clean tissue. Do not use water or other liquids to clean the mouthpiece.
• Always close the white protective cover of the inhaler when it is not being used.
• Protect the inhaler against moisture.
Other information about the inhaler
• The white protective cover will still twist and “click” even when the inhaler is empty.
• The sound heard when the inhaler is shaken is produced by a drying agent and not by the medicine. Therefore, sound does not tell you how much medicine is left in the inhaler.
• It is not possible to load the inhaler with more than one dose.
• If the device is dropped without the cap, then the cap should be replaced before the next dose is taken.
4.3 Contraindications
Hypersensitivity (allergy) to any of the active substances or to the excipient listed in section 6.1.
4.4 Special warnings and precautions for use
For patients with COPD experiencing exacerbations, treatment with systemic corticosteroids is typically indicated, therefore patients should be instructed to seek medical attention if symptoms deteriorate with Lifsar.
Treatment with Lifsar should not be stopped abruptly due to risk of exacerbation.
For patients with COPD cessation of therapy may also be associated with symptomatic decompensation and should be supervised by a physician.
As with all inhaled medication containing corticosteroids, Lifsar should be administered with caution in patients with active or quiescent pulmonary tuberculosis and fungal, viral or other infections of the airway. Appropriate treatment should be promptly instituted, if indicated.
Rarely, Lifsar may cause cardiac arrhythmias e.g. supraventricular tachycardia, extrasystoles and atrial fibrillation, and a mild transient reduction in serum potassium at high therapeutic doses. Therefore Lifsar should be used with caution in patients with severe cardiovascular disorders or heart rhythm abnormalities and patients with diabetes mellitus, thyrotoxicosis, uncorrected hypokalaemia or patients predisposed to low levels of serum potassium.
There have been very rare reports of increases in blood glucose levels (see section 4.8) and this should be considered when prescribing to patients with a history of diabetes mellitus.
As with other inhalation therapy paradoxical bronchospasm may occur with an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting bronchodilator and should be treated straightaway. Lifsar should be discontinued immediately, the patient assessed and alternative therapy instituted if necessary.
The pharmacological side effects of f>2 agonist treatment, such as tremor, palpitations and headache, have been reported, but tend to be transient and reduce with regular therapy.
Systemic effects may occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur than with oral corticosteroids. Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, decrease in bone mineral density, cataract and glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). It is important, therefore, that the patient is reviewed regularly and the dose of inhaled corticosteroid is reduced to the lowest dose at which effective control of the disease is maintained.
Prolonged treatment of patients with high doses of inhaled corticosteroids may result in adrenal suppression and acute adrenal crisis. Very rare cases of adrenal suppression and acute adrenal crisis have also been described with doses of fluticasone propionate between 500 and less than 1000 micrograms. Situations, which could potentially trigger acute adrenal crisis, include trauma, surgery, infection or any rapid reduction in dosage. Presenting symptoms are typically vague and may include anorexia, abdominal pain, weight loss, tiredness, headache, nausea, vomiting, hypotension, decreased level of consciousness, hypoglycaemia, and seizures. Additional systemic corticosteroid cover should be considered during periods of stress or elective surgery.
The benefits of inhaled fluticasone propionate therapy should minimise the need for oral steroids, but patients transferring from oral steroids may remain at risk of impaired adrenal reserve for a considerable time. Therefore these patients should be treated with special care and adrenocortical function regularly monitored. Patients who have required high dose emergency corticosteroid therapy in the past may also be at risk. This possibility of residual impairment should always be borne in mind in emergency and elective situations likely to produce stress, and appropriate corticosteroid treatment must be considered. The extent of the adrenal impairment may require specialist advice before elective procedures.
Ritonavir can greatly increase the concentration of fluticasone propionate in plasma. Therefore, concomitant use should be avoided, unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side-effects. There is also an increased risk of systemic side effects when combining fluticasone propionate with other potent CYP3A inhibitors (see section 4.5).
Pneumonia in patients with COPD
An increase in the incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. There is some evidence of an increased risk of pneumonia with increasing steroid dose but this has not been demonstrated conclusively across all studies. There is no conclusive clinical evidence for intra-class differences in the magnitude of the pneumonia risk among inhaled corticosteroid products. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia in patients with COPD include current smoking, older age, low body mass index (BMI) and severe COPD.
There was an increased reporting of lower respiratory tract infections (particularly pneumonia and bronchitis) in the TORCH study in patients with COPD receiving salmeterol/fluticasone propionate (FP) 50/500 micrograms bd. compared with placebo as well as in studies SCO40043 and SCO1000250 comparing the lower non-approved COPD dose of salmeterol/FP 50/250 micrograms bd., to salmeterol 50 micrograms bd. only (see sections 4.8 and 5.1). A similar incidence of pneumonia in the salmeterol/FP group was seen across all studies. If a patient with severe COPD has experienced pneumonia the treatment with Lifsar should be re-evaluated.
Data from a large clinical trial (the Salmeterol Multi-Center Asthma Research Trial, SMART) suggested African-American patients were at increased risk of serious respiratory-related events or deaths when using salmeterol compared with placebo (see section 5.1). It is not known if this was due to pharmacogenetic or other factors. Patients of black African or Afro-Caribbean ancestry should therefore be asked to continue treatment but to seek medical advice if asthma symptoms remained uncontrolled or worsen whilst using Lifsar.
Concomitant use of systemic ketoconazole significantly increases systemic exposure to salmeterol. This may lead to an increase in the incidence of systemic effects (e.g. prolongation in the QTc interval and palpitations). Concomitant treatment with ketoconazole or other potent CYP3A4 inhibitors should therefore be avoided unless the benefits outweigh the potentially increased risk of systemic side effects of salmeterol treatment (see section 4.5).
Lifsar contains lactose monohydrate up to 7 milligrams per metered dose. This amount does not usually cause problems in lactose intolerant people. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Beta adrenergic blockers may weaken or antagonise the effect of salmeterol. Both nonselective and selective P blockers should be avoided unless there are compelling reasons for their use. Potentially serious hypokalaemia may result from p2 agonist therapy. This effect may be potentiated by concomitant treatment with xanthine derivatives, steroids and diuretics.
Concomitant use of other p adrenergic containing drugs can have a potentially additive effect.
Fluticasone propionate
Under normal circumstances, low plasma concentrations of fluticasone propionate are achieved after inhaled dosing, due to extensive first pass metabolism and high systemic clearance mediated by cytochrome P450 3A4 in the gut and liver. Hence, clinically significant drug interactions mediated by fluticasone propionate are unlikely.
In an interaction study in healthy subjects with intranasal fluticasone propionate, ritonavir (a highly potent cytochrome P450 3A4 inhibitor) 100 mg bd. increased the fluticasone propionate plasma concentrations several hundred fold, resulting in markedly reduced serum cortisol concentrations. Information about this interaction is lacking for inhaled fluticasone propionate, but a marked increase in fluticasone propionate plasma levels is expected. Cases of Cushing's syndrome and adrenal suppression have been reported. The combination should be avoided unless the benefit outweighs the increased risk of systemic glucocorticoid side-effects.
In a small study in healthy volunteers, the slightly less potent CYP3A inhibitor ketoconazole increased the exposure of fluticasone propionate after a single inhalation by 150%. This resulted in a greater reduction of plasma cortisol as compared with fluticasone propionate alone. Co-treatment with other potent CYP3A inhibitors, such as itraconazole, and moderate CYP3A inhibitors, such as erythromycin, is also expected to increase the systemic fluticasone propionate exposure and the risk of systemic side-effects. Caution is recommended and longterm treatment with such drugs should if possible be avoided.
Salmeterol
Potent CYP3A4 inhibitors
Co-administration of ketoconazole (400 mg orally once daily) and salmeterol (50 micrograms inhaled twice daily) in 15 healthy subjects for 7 days resulted in a significant increase in plasma salmeterol exposure (1.4-fold Cmax and 15-fold AUC). This may lead to an increase in the incidence of other systemic effects of salmeterol treatment (e.g. prolongation of QTc interval and palpitations) compared with salmeterol or ketoconazole treatment alone (see section 4.4).
Clinically significant effects were not seen on blood pressure, heart rate, blood glucose and blood potassium levels. Co-administration with ketoconazole did not increase the elimination half-life of salmeterol or increase salmeterol accumulation with repeat dosing.
The concomitant administration of ketoconazole should be avoided, unless the benefits outweigh the potentially increased risk of systemic side effects of salmeterol treatment. There is likely to be a similar risk of interaction with other potent CYP3A4 inhibitors (e.g. itraconazole, telithromycin, ritonavir).
Moderate CYP3A4 inhibitors
Co-administration of erythromycin (500 mg orally three times a day) and salmeterol (50 micrograms inhaled twice daily) in 15 healthy subjects for 6 days resulted in a small but non-statistically significant increase in salmeterol exposure (1.4-fold Cmax and 1.2-fold AUC). Co-administration with erythromycin was not associated with any serious adverse effects.
4.6 Fertility, pregnancy and lactation
Fertility
There are no data in humans. However, animal studies showed no effects of salmeterol or fluticasone propionate on fertility.
Pregnancy
A moderate amount of data on pregnant women (between 300 - 1,000 pregnancy outcomes) indicates no malformative or feto/neonatal toxicity of salmeterol and fluticasone propionate. Animal studies have shown reproductive toxicity after administration of p2 adrenoreceptor agonists and glucocorticosteroids (see section 5.3).
Administration of Lifsar to pregnant women should only be considered if the expected benefit to the mother is greater than any possible risk to the fetus.
The lowest effective dose of fluticasone propionate needed to maintain adequate control of the disease should be used in the treatment of pregnant women.
Breastfeeding
It is unknown whether salmeterol and fluticasone propionate/metabolites are excreted in human milk.
Studies have shown that salmeterol and fluticasone propionate, and their metabolites, are excreted into the milk of lactating rats.
A risk to breastfed newborns/infants cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue Lifsar therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
4.7 Effects on ability to drive and use machines
Lifsar has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
As Lifsar contains salmeterol and fluticasone propionate, the type and severity of adverse reactions associated with each of the compounds may be expected. There is no incidence of additional adverse events following concurrent administration of the two compounds.
Adverse events which have been associated with salmeterol/fluticasone propionate are given below, listed by system organ class and frequency. Frequencies are defined as: very common (>1/10), common (>1/100 and <1/10), uncommon (>1/1000 and <1/100), rare (>1/10,000 to < 1/1000), very rare (<1/10,000) and not known (cannot be estimated from the available data). Frequencies were derived from clinical trial data. The incidence in placebo was not taken into account.
|
System Organ Class |
Adverse Event |
Frequency |
|
Infections and infestations |
Candidiasis of the mouth and throat |
Common |
|
Pneumonia (in COPD patients) |
Common1, 3 5 | |
|
Bronchitis |
Common1, 3 | |
|
Immune system disorders |
Hypersensitivity reactions with the following manifestations: - Cutaneous hypersensitivity reactions - Respiratory symptoms (dyspnoea) - Angioedema (mainly facial and oropharyngeal oedema) - Anaphylactic reactions including anaphylactic shock - Respiratory symptoms (bronchospasm) |
Uncommon Rare |
|
Endocrine disorders |
Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density |
Rare4 |
|
Metabolism and nutrition disorders |
Hypokalaemia |
Common3 |
|
Hyperglycaemia |
Uncommon4 | |
|
Psychiatric disorders |
Anxiety, sleep disorders |
Uncommon |
|
Behavioural changes, including psychomotor hyperactivity and irritability (predominantly in children) |
Rare | |
|
Depression, aggression (predominantly in children) |
Not known | |
|
Nervous system disorders |
Headache |
Very common1 |
|
Tremor |
Uncommon | |
|
Eye disorders |
Cataract |
Uncommon |
|
Glaucoma |
Rare4 | |
|
Cardiac disorders |
Palpitations, tachycardia, atrial fibrillation, angina pectoris |
Uncommon |
|
Cardiac arrhythmias (including supraventricular tachycardia and extrasystoles) |
Rare |
1 Reported commonly in placebo.
|
Respiratory, thoracic and mediastinal disorders |
Nasopharyngitis |
Very common2, 3 |
|
Throat irritation, hoarseness/dysphonia |
Common | |
|
Sinusitis |
Common1, 3 | |
|
Paradoxical bronchospasm |
Rare4 | |
|
Skin and subcutaneous tissue disorders |
Contusions |
Common1, 3 |
|
Musculoskeletal and connective tissue disorders |
Muscle cramps, arthralgia, myalgia |
Common |
|
Traumatic fractures |
Common1, 3 |
2 Reported very commonly in placebo.
3 Reported over 3 years in a COPD study.
4 See section 4.4.
5 See section 5.1.
Description of selected adverse reactions
The pharmacological side effects of p2 agonist treatment, such as tremor, palpitations and headache, have been reported, but tend to be transient and reduce with regular therapy.
As with other inhalation therapy paradoxical bronchospasm may occur with an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting bronchodilator and should be treated straightaway. Lifsar should be discontinued immediately, the patient assessed and alternative therapy instituted if necessary.
Due to the fluticasone propionate component, hoarseness and candidiasis (thrush) of the mouth and throat and, rarely, of the oesophagus can occur in some patients. Both hoarseness and incidence of candidiasis may be relieved by rinsing the mouth with water and/or brushing the teeth after using the product. Symptomatic mouth and throat candidiasis can be treated with topical anti-fungal therapy whilst still continuing with the Lifsar.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov .uk/yellowcard.
4.9 Overdose
There are no data available from clinical trials on overdose with Lifsar, however data on overdose with both drugs are given below:
The signs and symptoms of salmeterol overdose are dizziness, increase in systolic blood pressure, tremor, headache and tachycardia. If Lifsar therapy has to be withdrawn due to overdose of the P agonist component of the drug, provision of appropriate replacement steroid therapy should be considered. Additionally, hypokalaemia can occur and therefore serum potassium levels should be monitored. Potassium replacement should be considered.
Acute: Acute inhalation of fluticasone propionate doses in excess of those recommended may lead to temporary suppression of adrenal function. This does not need emergency action as adrenal function is recovered in a few days, as verified by plasma cortisol measurements.
Chronic overdose of inhaled fluticasone propionate: Refer to section 4.4: risk of adrenal suppression: Adrenal reserve should be monitored and treatment with a systemic corticosteroid may be necessary. When stabilised, treatment should be continued with an inhaled corticosteroid at the recommended dose.
In cases of both acute and chronic fluticasone propionate overdose, Lifsar therapy should be continued at a suitable dosage for symptom control.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Adrenergics, inhalants; Adrenergics in combination with corticosteroids or other drugs, excl. anticholinergics.
ATC code: R03AK06.
Mechanism of action and pharmacodynamic effects
Lifsar contains salmeterol and fluticasone propionate which have differing modes of action. The respective mechanisms of action of both drugs are discussed below:
Salmeterol
Salmeterol is a selective long-acting (12 hour) p2 adrenoceptor agonist with a long side chain which binds to the exo-site of the receptor.
Salmeterol produces a longer duration of bronchodilation, lasting for at least 12 hours, than recommended doses of conventional short-acting p2 agonists.
Fluticasone propionate (FP)
Fluticasone propionate given by inhalation at recommended doses has a glucocorticoid antiinflammatory action within the lungs, resulting in reduced symptoms and exacerbations of asthma, with less the adverse effects than when corticosteroids are administered systemically.
Clinical efficacy and safety
COPD clinical trials
TORCH was a 3-year study to assess the effect of treatment with salmeterol/FP 50/500 micrograms bd., salmeterol 50 micrograms bd., fluticasone propionate 500 micrograms bd. or placebo on all-cause mortality in patients with COPD. COPD patients with a baseline (pre-bronchodilator) FEVi < 60% of predicted normal were randomised to double-blind medication. During the study, patients were permitted usual COPD therapy with the exception of other inhaled corticosteroids, long-acting bronchodilators and long-term systemic corticosteroids. Survival status at 3 years was determined for all patients regardless of withdrawal from study medication. The primary endpoint was reduction in all-cause mortality at 3 years for salmeterol/FP vs. Placebo.
|
Placebo N = 1,524 |
Salmeterol 50 N = 1,521 |
FP 500 N = 1,534 |
Salmeterol + FP 50/500 N = 1,533 | |
|
All cause mortality at 3 years | ||||
|
Number of deaths (%) |
231 (15.2%) |
205 (13.5%) |
246 (16.0%) |
193 (12.6%) |
|
Hazard Ratio vs. Placebo (CIs) p value |
N/A |
0.879 (0.73, 1.06) 0.180 |
1.060 (0.89, 1.27) 0.525 |
0.825 (0.68, 1.00) 0.0521 |
|
Hazard Ratio Salmeterol + FP 50/500 vs. components (CIs) p value |
N/A |
0.932 (0.77, 1.13) 0.481 |
0.774 (0.64, 0.93) 0.007 |
N/A |
|
1 Non significant p value after adjustment for 2 interim analyses on the primary efficacy comparison from a log-rank analysis stratified by smoking status. | ||||
There was a trend towards improved survival in subjects treated with salmeterol/FP compared with placebo over 3 years however this did not achieve the statistical significance level p<0.05.
The percentage of patients who died within 3 years due to COPD-related causes was 6.0% for placebo, 6.1% for salmeterol, 6.9% for FP and 4.7% for salmeterol/FP.
The mean number of moderate to severe exacerbations per year was significantly reduced with salmeterol/FP as compared with treatment with salmeterol, FP and placebo (mean rate in the salmeterol/FP group 0.85 compared with 0.97 in the salmeterol group, 0.93 in the FP group and 1.13 in the placebo). This translates to a reduction in the rate of moderate to severe exacerbations of 25% (95% CI: 19% to 31%; p < 0.001) compared with placebo, 12% compared with salmeterol (95% CI: 5% to 19%, p = 0.002) and 9% compared with FP (95% CI: 1% to 16%, p = 0.024). Salmeterol and FP significantly reduced exacerbation rates compared with placebo by 15% (95% CI: 7% to 22%; p < 0.001) and 18% (95% CI: 11% to 24%; p < 0.001) respectively.
Health Related Quality of Life, as measured by the St George's Respiratory Questionnaire (SGRQ) was improved by all active treatments in comparison with placebo. The average improvement over three years for salmeterol/FP compared with placebo was -3.1 units (95% CI: -4.1 to -2.1; p < 0.001), compared with salmeterol was -2.2 units (p < 0.001) and compared with FP was -1.2 units (p = 0.017). A 4-unit decrease is considered clinically relevant.
The estimated 3-year probability of having pneumonia reported as an adverse event was 12.3% for placebo, 13.3% for salmeterol, 18.3% for FP and 19.6% for salmeterol/FP (Hazard ratio for salmeterol/FP vs. placebo: 1.64, 95% CI: 1.33 to 2.01, p < 0.001). There was no increase in pneumonia related deaths; deaths while on treatment that were adjudicated as primarily due to pneumonia were 7 for placebo, 9 for salmeterol, 13 for FP and 8 for salmeterol/FP. There was no significant difference in probability of bone fracture (5.1% placebo, 5.1% salmeterol, 5.4% FP and 6.3% salmeterol/FP; Hazard ratio for salmeterol/FP vs. placebo: 1.22, 95% CI: 0.87 to 1.72, p = 0.248).
Placebo-controlled clinical trials, over 6 and 12 months, have shown that regular use of salmeterol/FP 50/500 micrograms improves lung function and reduces breathlessness and the use of relief medication.
Studies SCO40043 and SCO100250 were randomised, double-blind, parallel-group, replicate studies comparing the effect of salmeterol/FP 50/250 micrograms bd. (a dose not licensed for
COPD treatment in the European Union) with salmeterol 50 micrograms bd. on the annual rate of moderate/severe exacerbations in subjects with COPD with FEV1 less than 50% predicted and a history of exacerbations. Moderate/severe exacerbations were defined as worsening symptoms that required treatment with oral corticosteroids and/or antibiotics or in-patient hospitalisation.
The trials had a 4 week run-in period during which all subjects received open-label salmeterol/FP 50/250 to standardize COPD pharmacotherapy and stabilise disease prior to randomisation to blinded study medication for 52 weeks. Subjects were randomised 1:1 to salmeterol/FP 50/250 (total ITT n = 776) or salmeterol (total ITT n = 778). Prior to run-in, subjects discontinued use of previous COPD medications except short-acting bronchodilators. The use of concurrent inhaled long-acting bronchodilators (p2 agonist and anticholinergic), ipratropium/salbutamol combination products, oral p2 agonists, and theophylline preparations were not allowed during the treatment period. Oral corticosteroids and antibiotics were allowed for the acute treatment of COPD exacerbations with specific guidelines for use. Subjects used salbutamol on an as-needed basis throughout the studies.
The results of both studies showed that treatment with salmeterol/FP 50/250 resulted in a significantly lower annual rate of moderate/severe COPD exacerbations compared with salmeterol (SCO40043: 1.06 and 1.53 per subject per year, respectively, rate ratio of 0.70, 95% CI: 0.58 to 0.83, p < 0.001; SCO100250: 1.10 and 1.59 per subject per year, respectively, rate ratio of 0.70, 95% CI: 0.58 to 0.83, p < 0.001). Findings for the secondary efficacy measures (time to first moderate/severe exacerbation, the annual rate of exacerbations requiring oral corticosteroids, and pre-dose morning (AM) FEV1) significantly favoured salmeterol/FP 50/250 micrograms bd. over salmeterol. Adverse event profiles were similar with the exception of a higher incidence of pneumonias and known local side effects (candidiasis and dysphonia) in the salmeterol/FP 50/250 micrograms bd. group compared with salmeterol. Pneumonia-related events were reported for 55 (7%) subjects in the salmeterol/FP 50/250 micrograms bd. group and 25 (3%) in the salmeterol group. The increased incidence of reported pneumonia with salmeterol/FP 50/250 micrograms bd. appears to be of similar magnitude to the incidence reported following treatment with salmeterol/FP 50/500 micrograms bd. in TORCH.
5.2 Pharmacokinetic properties
For pharmacokinetic purposes each component can be considered separately.
Salmeterol
Salmeterol acts locally in the lung therefore plasma levels are not an indication of therapeutic effects. In addition there are only limited data available on the pharmacokinetics of salmeterol because of the technical difficulty of assaying the drug in plasma due to the low plasma concentrations at therapeutic doses (approximately 200 picogram/ml or less) achieved after inhaled dosing.
Fluticasone propionate
The absolute bioavailability of a single dose of inhaled fluticasone propionate in healthy subjects varies between approximately 5 to 11% of the nominal dose depending on the inhalation device used. In patients with asthma or COPD a lesser degree of systemic exposure to inhaled fluticasone propionate has been observed.
Systemic absorption occurs mainly through the lungs and is initially rapid then prolonged.
The remainder of the inhaled dose may be swallowed but contributes minimally to systemic
exposure due to the low aqueous solubility and pre-systemic metabolism, resulting in oral availability of less than 1%. There is a linear increase in systemic exposure with increasing inhaled dose.
The disposition of fluticasone propionate is characterised by high plasma clearance (1150 ml/min), a large volume of distribution at steady-state (approximately 300 l) and a terminal half-life of approximately 8 hours.
Plasma protein binding is 91%.
Fluticasone propionate is cleared very rapidly from the systemic circulation. The main pathway is metabolism to an inactive carboxylic acid metabolite, by the cytochrome P450 enzyme CYP3A4. Other unidentified metabolites are also found in the faeces.
The renal clearance of fluticasone propionate is negligible. Less than 5% of the dose is excreted in urine, mainly as metabolites. The main part of the dose is excreted in faeces as metabolites and unchanged drug.
5.3 Preclinical safety data
The only safety concerns for human use derived from animal studies of salmeterol xinafoate and fluticasone propionate given separately were effects associated with exaggerated pharmacological actions.
In animal reproduction studies, glucocorticosteroids have been shown to induce malformations (cleft palate, skeletal malformations). However, these animal experimental results do not seem to be relevant for man given recommended doses. Animal studies with salmeterol xinafoate have shown embryofetal toxicity only at high exposure levels. Following co-administration, increased incidences of transposed umbilical artery and incomplete ossification of occipital bone were found in rats at doses associated with known glucocorticoid-induced abnormalities.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Lactose monohydrate.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
Shelf life after first opening: 30 days.
6.4 Special precautions for storage
Keep the cover tightly closed in order to protect from moisture.
6.5 Nature and contents of container
Each carton box contains one PulmoJet inhaler with 60 inhalations. The inhaler body is grey and white with a grey base and mouthpiece, a white cover and a purple or grey bottom lid and is made of six different plastic materials: polypropylene/polyethylene/acrylonitrile butadiene styrene/thermoplastic elastomer/polybutylene terephthalate and silicon.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Winthrop Pharmaceuticals UK Limited
One Onslow Street
Guildford
Surrey
GU1 4YS
United Kingdom
Trading as:
Zentiva, One Onslow Street, Guildford, Surrey, GU1 4YS, UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 17780/0673
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
05/08/2015
10 DATE OF REVISION OF THE TEXT
17/11/2016