Lisvy 60 Micrograms/24 Hours + 13 Micrograms/24 Hours Transdermal Patch
SUMMARY OF PRODUCT CHARACTERISTICS
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
1 NAME OF THE MEDICINAL PRODUCT
Lisvy 60 micrograms/24 hours + 13 micrograms/24 hours_transdermal patch
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 11 cm transdermal patch contains 2.10 mg gestodene and 0.55 mg ethinylestradiol.
Each transdermal patch releases 60 micrograms gestodene per 24 hours and 13 micrograms ethinylestradiol (equal to oral doses of 20 micrograms) per 24 hours.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Transdermal patch.
Thin matrix-type transdermal patch consisting of five layers.
The patch is round, transparent, and has a size of 11 cm2. On its sticky side the patch is covered by a two-part square-shaped shiny clear protective liner.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Female hormonal contraception.
apleek is intended for women of fertile age. Safety and efficacy have been established in women aged 18 to 45 years.
The decision to prescribe apleek should take into consideration the individual woman’s current risk factors, particularly those for venous thromboembolism (VTE), and how the risk of VTE with apleek compares with other combined hormonal contraceptives (CHCs) (see sections 4.3 and 4.4).
4.2 Posology and method of administration
Posolosv
Lisvy is used in a 28-day (4-week) cycle:
For three consecutive weeks (21 days), one new patch is applied per week and the used patch is removed. Week Four is patch-free. Withdrawal bleeding is expected to begin during this time. One week after the last patch is removed, a new 4-week cycle is started by applying a new patch (on the same day of the week as before, the ‘Patch Change Day’), regardless of whether usual withdrawal bleeding is continuing or has stopped. See ‘Reduced cycle control’ in section 4.4 in case the usual withdrawal bleed does not occur. For the precise scheme when to apply/remove a patch, see ‘Patch Change Day’ in section ‘How to use Lisvy’.
When to start Lisvy for the first time
• No preceding hormonal contraceptive use (in the past month)
The patch has to be applied on the first day of the woman’s natural cycle (i.e., the first day of her menstrual bleeding). Starting on Days 2-5 is allowed, but during the first cycle a barrier method is required during the 7 days of wearing the first patch.
• Changing from a combined hormonal contraceptive (combined oral contraceptive (COC), vaginal ring, or a different transdermal patch)
The patch should be applied preferably on the day after the last hormone-containing COC tablet, but at the latest on the day following the usual tablet-free or hormone-free tablet interval of the COC.
When switching from a vaginal ring or a different transdermal patch, the woman should apply the patch preferably on the day of removal of the last ring or patch of a cycle pack, but at the latest when the next application would have been due.
• Changing from a progestogen-only-method (progestogen-only pill, injection, implant) or from a progestogen-releasing intrauterine system (IUS)
The woman may switch any day from the progestogen-only pill (from an implant or an IUS, on the day of its removal; or from an injectable, when the next injection would be due). In all of these cases, the woman should be advised to additionally use a barrier method during the 7 days of wearing the first patch.
• Following first-trimester abortion
The woman may start immediately. When doing so, she does not need to take additional contraceptive measures.
• Following delivery or second-trimester abortion
Women should be advised to start during Days 21 to 28 after delivery or second-trimester abortion. When starting later, the woman should be advised to additionally use a barrier method during the 7 days of wearing the first patch. However, if intercourse has already occurred, pregnancy should be excluded before the actual start of Lisvy or the woman has to wait for her first menstrual period.
For breastfeeding women, see section 4.6.
How to use Lisvy
Lisvy is used in a 28-day (4-week) cycle (1 patch a week for 3 weeks followed by a 7-day patch-free interval). Only one patch is to be worn at a time. Each subsequent cycle starts immediately after the patch-free interval of the previous cycle regardless of whether usual withdrawal bleeding is continuing or has stopped.
• Patch Change Day
Every new patch should be applied on the same day of the week. This day is known as the 'Patch Change Day'. For example, if the first patch is applied on a Sunday, all subsequent patches should be applied on a Sunday. Only one patch is to be worn at a time.
1st patch 2nd patch
3rd patch No patch
Day 1: application of 1st patch (for women starting new with Lisvy, refer to ‘When to start Lisvy for the first time’)
Day 8: removal of 1st patch and immediate application of the 2nd patch
Day 15: removal of 2nd patch and immediate application of the
3rd patch
Day 22: removal of 3rd patch (no patch from Days 22-28)
Patch removal occurs on the same day of the week (‘Patch Change Day’). Patch changes may occur at any time on the ‘Patch Change Day’. Subsequent cycles are started on the same ‘Patch Change Day’, after the 7-day patch-free interval (Days 22-28).
• Patch Free Days
rd
No patch is worn from Day 22 (after removal of the 3 patch) through Day 28 (‘Week Four’).
Under no circumstances should there be more than a 7-day patch-free interval between cycles.
If there are more than seven patch-free days, THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY. A new cycle must be started by applying a new patch as soon as the woman has noticed the missed start of a new cycle and back-up contraception, such as condoms, spermicide, or diaphragm, must be used for the next seven days. As with COCs, the risk of ovulation increases with each day beyond the recommended drug-free period.
If intercourse has occurred during such an extended patch-free interval, the possibility of pregnancy should be considered.
See also ‘Management of detached, missed, or not replaced patches’.
Method of administration
Route of administration: Transdermal use
Where to apply the patch
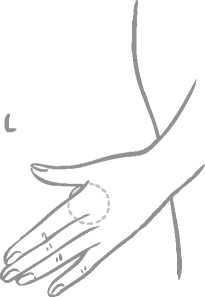
A patch should only be applied to any of the following application sites (see figure below): abdomen, buttocks, outer upper arm.
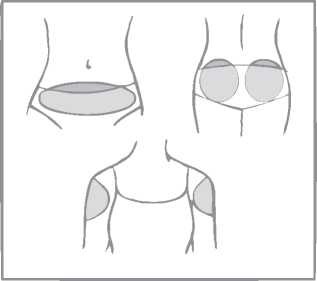
Areas where the patch may be rubbed off (e.g. waistband of clothing) should be avoided.
The patch should be applied to clean, dry, intact, healthy, and preferably hairless skin.
Lisvy should not be placed on skin that is oily, red, irritated, cut or otherwise damaged.
Patches must not be applied to the breasts.
To prevent interference with the adhesive properties of Lisvy, no make-up, creams, lotions, powders or other topical products should be applied to the skin area where Lisvy is or will be placed.
Application spots should be varied. This can be done by using different areas at the same application site. For example, the woman can change from left to right side of the abdomen or from left to right buttock or upper arm.
The woman can also use a different application site each week (for example one week the outer upper arm, next week the abdomen).
The woman should visually check her patch daily to ensure continued proper adhesion._
Please note
Only one patch is to be worn at a time.
If the patch is applied correctly, the woman can bath or shower as usual.
The transparent patch is UV/sunlight protected so it can be exposed to sunlight and does not need to remain covered by clothing.
In case of skin irritation
If patch use results in uncomfortable irritation at the site of the patch, the patch is to be removed and a new patch is to be placed on a different location. That patch is used until the next scheduled ‘Patch Change Day’.
How to prepare the patch for application
Lisvy is contained in a box that includes: a booklet plus either 3 or 9 or 18 sealed sachets, each containing one Lisvy transdermal patch.
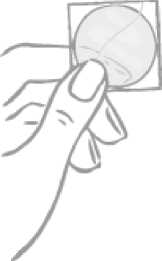
The patch is round and transparent:
On its sticky side the patch is covered by a two-part square-shaped shiny clear protective liner. This liner protects the sticky side that contains the active components of the patch. It also ensures that the sticky surface will be maintained until application.
On the opposite side, the patch is covered by a milky white square-shaped cover sheet which prevents the patch from getting stuck in the sachet.
Round transparent patch plus two-part square-shaped, shiny, clear protective liner
Milky white square-shaped cover sheet
The woman should tear along the top side of the sachet with her fingers. The notches will help guide the tear.
The woman should not use scissors nor cut, damage or alter the patch in any way because this may reduce the contraceptive effect.
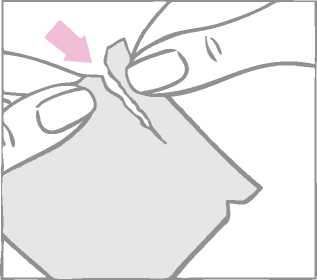
The round contraceptive patch is enclosed between a two-part square-shaped shiny clear protective liner and a milky-white square shaped cover sheet. It is important to remove the patch together with both the clear protective liner and the milky-white cover sheet from the sachet. The sachet should not be discarded. It should be kept for disposal of the patch after use.
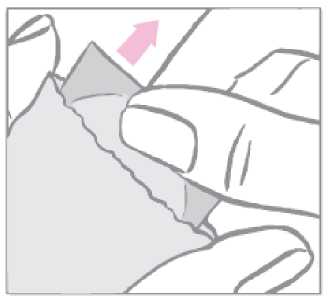
The patch is to be applied immediately after opening the sachet, as follows:
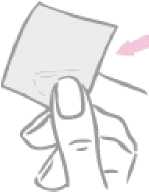
First the woman removes the one-piece milky white square-shaped cover sheet from the top side of the patch.
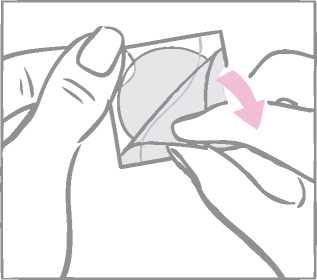
After removal from the patch, the milky white square-shaped cover sheet that prevents the patch from getting stuck in the pouch should be discarded.
Next the woman removes half of the two-part, square-shaped shiny clear protective liner that covers the bottom (sticky) side of the round transparent patch. (The sticky side contains the active drugs.) She should avoid touching the sticky surface of the patch so that the stickiness is maintained.
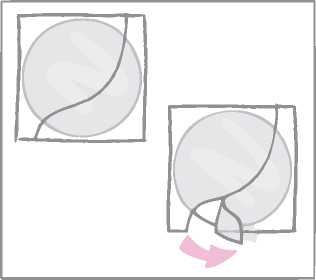
While holding the patch by the edge which is still covered by the second half of the protective liner, the patch should be positioned on the skin where it will be worn.
With half of the patch gently sticking to the application site, the second half of the protective liner should be removed.
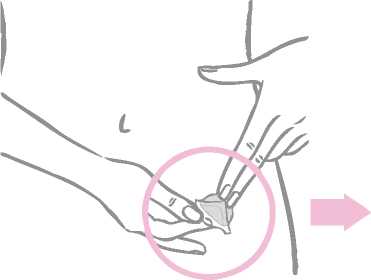
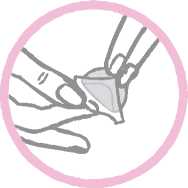
The woman should press down firmly on the patch with the palm of her hand for 30 seconds and should make sure that the edges stick well.
Note: the sachet should not be discarded as it will be needed to dispose of the patch after use.
Disposal of patches See section 6.6.
Management of detached, missed, or not replaced patches
Management of patch application deviations is based on the following rule:
At least 7 consecutive days with a correctly applied patch are necessary to adequately suppress the hypothalamic-pituitary-ovarian axis as a basis for contraceptive efficacy.
If a patch is partially or completely detached: for less than one day (up to 24 hours)
The woman should try to reapply it to the same place or replace it with a new patch immediately. No back-up contraception is needed. The woman’s ‘Patch Change Day’ will remain the same.
for more than one day (24 hours or more),
or if the woman is not sure how long the patch has been detached:
SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new cycle immediately by applying a new patch. There is now a new ‘Day 1’ and a new ‘Patch Change Day’. Back-up contraception, such as condoms, spermicide, or diaphragm, must be used for the first week of the new cycle.
A patch should not be reapplied if it is no longer sticky, if it has become stuck to itself or another surface, if it has other material stuck to it or if it has previously become loose or fallen off. If a patch cannot be reapplied, a new patch should be applied immediately. Supplemental adhesives or wraps should not be used to hold Lisvy in place.
If the woman forgets to change her patch
at the start of any patch cycle (Week One /Day 1):
SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should apply the first patch of her new cycle as soon as she remembers. There is now a new
‘Patch Change Day’ and a new ‘Day 1’. The woman must use back-up contraception, such as condoms, spermicide, or diaphragm, for the first week of the new cycle.
in the middle of the patch cycle (Week Two/Day 8 or Week Three/Day 15): for one or two days (up to 48 hours),
she should apply a new patch immediately. The next patch should be applied on the usual ‘Patch Change Day’. No back-up contraception is needed.
for more than two days (48 hours or more),
SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new 4-week cycle immediately by putting on a new patch. There is now a new ‘Patch Change Day’ and a new ‘Day 1’. The woman must use back-up contraception for one week.
at the end of the patch cycle (Week Four/Day 22):
If the woman forgets to remove her patch on Day 22, she should take it off as soon as she remembers (at the latest by Day 28). The next cycle then must be started with a new patch (on the day after DAY 28 - the usual ‘Patch Change Day’), never later. No back-up contraception is needed.
Consequences of detached, missed, or not replaced patches and required action:
Consequences of detached, missed, or not replaced patches and required action
|
Detached patches a |
Time frame |
Consequences on contraceptive reliability a |
Required action a |
|
Patch detached |
< 24 hours |
Contraceptive efficacy ensured |
- Apply new patch immediately - No back-up contraception needed - ‘Patch Change Day’ unchanged |
|
> 24 hours |
Contraceptive efficacy compromised |
- Start new 4-week cycle immediately by applying a new patch - Use back-up contraception for the next 7 days b - Note new ‘Patch Change Day’ |
|
Patches not replaced on time a |
Time frame |
Consequences on contraceptive reliability a |
Required action a |
|
1st patch (Week 1, Day 1) not applied on time |
Patch-free interval d > 7 days |
Contraceptive efficacy compromised |
- Start new 4-week cycle immediately by applying a new patch - Use back-up contraception for the next 7 days b - Note new ‘Patch Change Day’ |
|
1st or 2nd patch (Week 1/2 or 2/3) not replaced on time |
< 48 hours |
Contraceptive efficacy ensured |
- Apply new patch immediately - No back-up contraception needed - ‘Patch Change Day’ unchanged |
|
> 48 hours |
Contraceptive efficacy compromised |
- Start new 4-week cycle immediately by applying a new patch - Use back-up contraception for the next 7 days b - Note new ‘Patch Change Day’ | |
|
3rd patch (Week 3/4) not removed on time |
Contraceptive efficacy ensured c |
- Remove patch - Start next 4-week cycle on your normal ‘Patch Change Day’ |
a Applies to each cycle.
b Back-up contraception is any additional non-hormonal method of contraception with the exception of the calendar method and the temperature method.
c Provided that the 3rd patch has been replaced by a new one at the latest on the regular Day 1 of the new patch cycle.
d Time since removal of last patch of the previous cycle._
The prescription of the next pack should be issued in time i.e. prior to the use of the last patch in the pack to ensure that the woman does not run out of patches.
‘Patch Change Day’ adjustment
If the woman wishes to change her ‘Patch Change Day’ she should complete her current cycle, removing the third patch on the correct day. During the patch-free week, she may select an earlier ‘Patch Change Day’ by applying a new patch on the desired day. In no case should there be more than 7 consecutive patch-free days.
Special populations
Gender
Lisvy is only indicated in women.
Elderly women
Not applicable. Lisvy is not indicated after menopause.
Body mass index
2
Data on contraceptive efficacy in women with Body Mass Index > 30 kg/m are limited.
Renal impairment
Lisvy has not been studied in women with renal impairment. No increased risk for women with renal impairment is expected (see section 5.2).
Hepatic impairment
Lisvy has not been studied in women with hepatic impairment. Lisvy is contraindicated in women with presence or history of severe hepatic disease as long as liver function values have not returned to normal. See also section 4.3.
Ethnic differences
The pharmacokinetics of ethinylestradiol was studied in combination with another progestin in Caucasian, Chinese and Japanese women and did not reveal any clinically significant difference. The pharmacokinetics of Lisvy has not been specifically studied in women of different ethnicities. No polymorphic enzymes are known that contribute to a major extent in the metabolization of gestodene. The available data in Caucasian, Black and Hispanic women do not indicate any relevant difference in the pharmacokinetics of Lisvy between different races/ethnicities. Very limited data is available for Asian women.
Paediatric population
Safety and efficacy have not been established in adolescents under 18 years of age. There is no relevant use of Lisvy in children and pre-menarcheal adolescents.
4.3 Contraindications
Combined hormonal contraceptives (CHCs) should not be used in the following conditions. Should any of the conditions appear for the first time during use of apleek, the patch should be removed immediately.
• Presence or risk of venous thromboembolism (VTE)
o Venous thromboembolism - current VTE (on anticoagulants) or history of (e.g. deep venous thrombosis [DVT] or pulmonary embolism [PE])
o Known hereditary or acquired predisposition for venous
thromboembolism, such as APC-resistance (including Factor V Leiden), antithrombin-III-deficiency, protein C deficiency, protein S deficiency
o Major surgery with prolonged immobilisation (see section 4.4)
o A high risk of venous thromboembolism due to the presence of multiple risk factors (see section 4.4).
• Presence or risk of arterial thromboembolism (ATE)
o Arterial thromboembolism - current arterial thromboembolism, history of arterial thromboembolism (e.g. myocardial infarction) or prodromal condition (e.g. angina pectoris)
o Cerebrovascular disease - current stroke, history of stroke or prodromal condition (e.g. transient ischaemic attack, TIA)
o Known hereditary or acquired predisposition for arterial
thromboembolism, such as hyperhomocysteinaemia and antiphospholipid-antibodies (anticardiolipin-antibodies, lupus anticoagulant)
o History of migraine with focal neurological symptoms
o A high risk of arterial thromboembolism due to multiple risk factors (see section 4.4) or to the presence of one serious risk factor such as:
• diabetes mellitus with vascular symptoms
• severe hypertension
• severe dyslipoproteinaemia
• Presence or history of severe hepatic disease as long as liver function values have not returned to normal.
• Presence or history of liver tumours (benign or malignant).
• Known or suspected sex-steroid influenced malignancies (e.g. of the genital organs or the breasts).
• Undiagnosed vaginal bleeding.
• Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Warnings
If any of the conditions or risk factors mentioned below is present, the suitability of apleek should be discussed with the woman. In the event of aggravation or first appearance of any of these conditions or risk factors, the woman should be advised to contact her doctor to determine whether the use of apleek should be discontinued.
• Circulatory Disorders
Risk of venous thromboembolism (VTE)
The use of any combined hormonal contraceptive (CHC) increases the risk of venous thromboembolism (VTE) compared with no use. Products that contain levonorgestrel, norgestimate or norethisterone are associated with the lowest risk of VTE. It is not yet known how the risk with apleek compares with these lower risk products. The decision to use any product other than one known to have the lowest VTE risk should be taken only after a discussion with the woman to ensure she understands the risk of VTE with CHCs, how her current risk factors influence this risk, and that her VTE risk is highest in the first ever year of use. There is also some evidence that the risk is increased when a CHC is restarted after a break in use of 4 weeks or more.
In women who do not use a CHC and are not pregnant about 2 out of 10,000 will develop a VTE over the period of one year. However, in any individual woman the risk may be far higher, depending on her underlying risk factors (see below).
It is estimated1 that out of 10,000 women who use a COC containing gestodene between 9 and 12 women will develop a VTE in one year; this compares with about 62 in women who use a levonorgestrel-containing CHC.
In both cases, the number of VTEs per year is fewer than the number expected during pregnancy or in the postpartum period.
It is not yet known how the risk of VTE with apleek compares with the risk with low dose levonorgestrel-containing CHCs and COCs containing gestodene.
VTE may be fatal in 1-2% of cases.
Number of VTE events per 10,000 women in one year
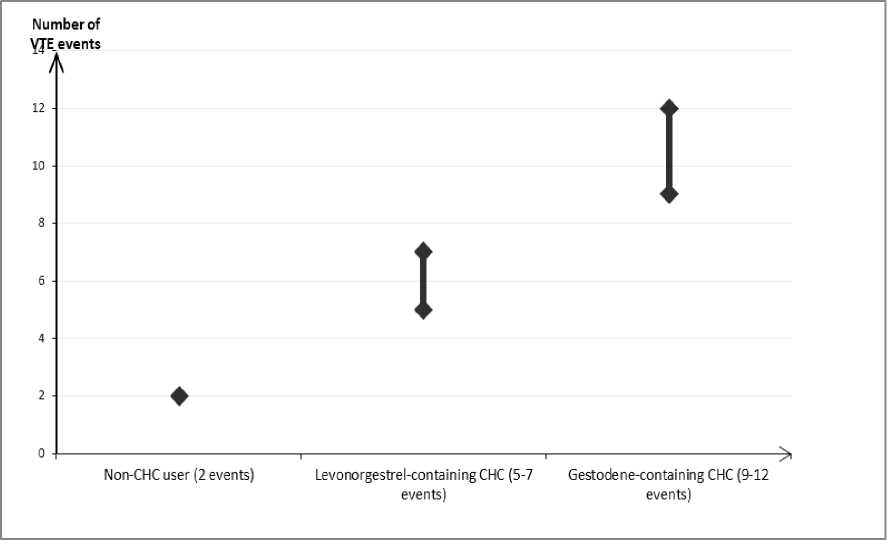
Extremely rarely, thrombosis has been reported to occur in CHC users in other blood vessels, e.g. hepatic, mesenteric, renal or retinal veins and arteries.
Risk factors for VTE
The risk for venous thromboembolic complications in CHC users may increase substantially in a woman with additional risk factors, particularly if there are multiple risk factors (see table).
apleek is contraindicated if a woman has multiple risk factors that put her at high risk of venous thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors - in this case her total risk of VTE should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3).
Table: Risk factors for VTE
|
Risk factor |
Comment |
|
Obesity (body mass index over 30 kg/m2) |
Risk increases substantially as BMI rises. Particularly important to consider if other risk factors also present. |
|
Prolonged immobilisation, major surgery, any surgery to the legs or pelvis, neurosurgery, or major trauma Note: temporary immobilisation including air travel >4 hours can also be a risk factor for VTE, particularly in women with other risk factors |
In these situations it is advisable to discontinue use of the patch (in the case of elective surgery at least four weeks in advance) and not resume until two weeks after complete remobilisation. Another method of contraception should be used to avoid unintentional pregnancy. Antithrombotic treatment should be considered if apleek has not been discontinued in advance. |
|
Positive family history (venous thromboembolism ever in a sibling or parent especially at a relatively early age e.g. before 50). |
If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any CHC use |
|
Other medical conditions associated with VTE |
Cancer, systemic lupus erythematosus, haemolytic uraemic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis) and sickle cell disease |
|
Increasing age |
Particularly above 35 years |
There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in the onset or progression of venous thrombosis.
The increased risk of thromboembolism in pregnancy, and particularly the 6 week period of the puerperium, must be considered (for information on “Pregnancy and lactation” see section 4.6).
Symptoms of VTE (deep vein thrombosis and pulmonary embolism)
In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is using a CHC.
Symptoms of deep vein thrombosis (DVT) can include:
- unilateral swelling of the leg and/or foot or along a vein in the leg;
- pain or tenderness in the leg which may be felt only when standing or walking;
- increased warmth in the affected leg; red or discoloured skin on the leg. Symptoms of pulmonary embolism (PE) can include:
- sudden onset of unexplained shortness of breath or rapid breathing;
- sudden coughing which may be associated with haemoptysis;
- sharp chest pain;
severe light headedness or dizziness; rapid or irregular heartbeat.
Some of these symptoms (e.g. "shortness of breath", "coughing") are non-specific and might be misinterpreted as more common or less severe events (e.g. respiratory tract infections).
Other signs of vascular occlusion can include: sudden pain, swelling and slight blue discoloration of an extremity.
If the occlusion occurs in the eye symptoms can range from painless blurring of vision which can progress to loss of vision. Sometimes loss of vision can occur almost immediately.
Risk of arterial thromboembolism (ATE)
Epidemiological studies have associated the use of CHCs with an increased risk for arterial thromboembolism (myocardial infarction) or for cerebrovascular accident (e.g. transient ischaemic attack, stroke). Arterial thromboembolic events may be fatal.
Risk factors for ATE
The risk of arterial thromboembolic complications or of a cerebrovascular accident in CHC users increases in women with risk factors (see table). apleek is contraindicated if a woman has one serious or multiple risk factors for ATE that puts her at high risk of arterial thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors - in this case her total risk should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3).
Table: Risk factors for ATE
|
Risk factor |
Comment |
|
Increasing age |
Particularly above 35 years |
|
Smoking |
Women should be advised not to smoke if they wish to use a CHC. Women over 35 who continue to smoke should be strongly advised to use a different method of contraception. |
|
Hypertension | |
|
Obesity (body mass index over 30 kg/m2) |
Risk increases substantially as BMI increases. Particularly important in women with additional risk factors |
|
Positive family history (arterial thromboembolism ever in a sibling or parent especially at relatively early age e.g. below 50). |
If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any CHC use |
|
Migraine |
An increase in frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation |
Other medical conditions associated with adverse vascular events
Diabetes mellitus, hyperhomocysteinaemia, valvular heart disease and atrial fibrillation, dyslipoproteinaemia and systemic lupus erythematosus.
Symptoms of ATE
In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is using a CHC.
Symptoms of a cerebrovascular accident can include:
- sudden numbness or weakness of the face, arm or leg, especially on one side of the body;
- sudden trouble walking, dizziness, loss of balance or coordination;
- sudden confusion, trouble speaking or understanding;
- sudden trouble seeing in one or both eyes;
- sudden, severe or prolonged headache with no known cause;
- loss of consciousness or fainting with or without seizure.
Temporary symptoms suggest the event is a transient ischaemic attack (TIA). Symptoms of myocardial infarction (MI) can include:
- pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm, or below the breastbone;
- discomfort radiating to the back, jaw, throat, arm, stomach;
- feeling of being full, having indigestion or choking;
- sweating, nausea, vomiting or dizziness;
- extreme weakness, anxiety, or shortness of breath;
- rapid or irregular heartbeats.
• Tumours
An increased risk of cervical cancer in long-term users of COCs (> 5 years) has been reported in some epidemiological studies, but there continues to be controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as human papilloma virus (HPV).
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using COCs. The excess risk gradually disappears during the course of the 10 years after cessation of COC use. Because breast cancer is rare in women under 40 years of age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer. These studies do not provide evidence for causation. The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The breast cancers diagnosed in ever-users tend to be less advanced clinically than the cancers diagnosed in never-users.
In rare cases, benign liver tumours, and even more rarely, malignant liver tumours have been reported in users of COCs. In isolated cases, these tumours have led to life-threatening intra-abdominal haemorrhages. A liver tumour should be considered in the differential diagnosis when severe upper abdominal pain, liver enlargement or signs of intra-abdominal haemorrhage occur in women using combined hormonal contraceptives.
With the use of COCs containing more than 50 pg ethinylestradiol, the risk of endometrial and ovarian cancer is reduced. Whether this also applies to lower-dosed combined hormonal contraceptives remains to be confirmed.
• Other conditions
If there are, repeatedly, persistent skin irritations (e.g. persistent erythema or pruritus at the application site) even if the application site is changed according to the directions one should consider discontinuation of transdermal treatment.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using combined hormonal contraceptives.
Although small increases in blood pressure have been reported in many women taking combined hormonal contraceptives, clinically relevant increases are rare. However, if a sustained clinically significant hypertension develops during the use of apleek then it is prudent for the physician to withdraw the preparation and treat the hypertension. Where considered appropriate, use of apleek may be resumed if normotensive values can be achieved with antihypertensive therapy.
The following conditions have been reported to occur or deteriorate with both pregnancy and combined hormonal contraceptive use, but the evidence of an association with combined hormonal contraceptive use is inconclusive: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; haemolytic uremic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosis-related hearing loss.
In women with hereditary angioedema exogenous oestrogens may induce or exacerbate symptoms of angioedema.
Acute or chronic disturbances of liver function may necessitate the discontinuation of apleek until markers of liver function return to normal. Recurrence of cholestatic jaundice which occurred first during pregnancy or previous use of sex steroids necessitates the discontinuation of apleek.
Although combined hormonal contraceptives may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for a need to alter the therapeutic regimen in diabetics using low-dose combined hormonal contraceptives (containing < 0.05 mg ethinylestradiol). However, diabetic women should be carefully observed while using combined hormonal contraceptives.
Worsening of endogenous depression, of epilepsy, of Crohn’s disease and of ulcerative colitis has been reported during combined hormonal contraceptives use.
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst using combined hormonal contraceptives.
Special attention should be paid to the interaction of combined hormonal contraceptives with lamotrigine (see section 4.5).
Medical examination/consultation
Prior to the initiation or reinstitution of apleek a complete medical history (including family history) should be taken and pregnancy must be ruled out. Blood pressure should be measured and a physical examination should be performed, guided by the contraindications (see section 4.3) and warnings (see section 4.4). It is important to
draw a woman’s attention to the information on venous and arterial thrombosis, including the risk of apleek compared with other CHCs, the symptoms of VTE and ATE, the known risk factors and what to do in the event of a suspected thrombosis.
The woman should also be instructed to carefully read the user leaflet and to adhere to the advice given. The frequency and nature of examinations should be based on established practice guidelines and be adapted to the individual woman.
Women should be advised that hormonal contraceptives do not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Reduced efficacy
The efficacy of apleek may be reduced, for example, in the event of
- missing the scheduled application of a patch
- a patch becoming detached
- a change of patch being forgotten (see ‘Management of detached, missed, or not replaced patches’ in section 4.2)
- concomitant medication (see section 4.5).
Reduced cycle control
With all combined hormonal contraceptives, irregular bleeding (spotting or breakthrough bleeding) may occur, especially during the first months of use. In such events, the application of apleek should be continued. The evaluation of any irregular bleeding is only meaningful after an adaptation interval of about three cycles of apleek use. The percentage of women using apleek experiencing intracyclic bleeding after this adaptation period ranged from 7 - 12 %.
Only a minority of women, in the range of 1% per cycle, were amenorrhoic.
If bleeding irregularities persist or occur after regular cycles of apleek, then nonhormonal causes should be considered and adequate diagnostic measures are indicated to exclude pregnancy or malignancy. These may include curettage.
In some women withdrawal bleeding may not occur during the patch-free interval. If apleek has been used according to the directions described in section 4.2, it is unlikely that the woman is pregnant. However, if apleek has not been used according to these directions prior to the first missed withdrawal bleed or if two withdrawal bleeds are missed, pregnancy must be ruled out before use of apleek is continued.
4.5 Interaction with other medicinal products and other forms of interaction
Note: The prescribing information of concomitant medications should be consulted to identify potential interactions.
Effects of other medicinal products on Lisvy
Interactions can occur with drugs that induce microsomal enzymes which can result in increased clearance of sex hormones and which may lead to breakthrough bleeding and/or contraceptive failure.
Management
Enzyme induction can already be observed after a few days of treatment. Maximal enzyme induction is generally seen within a few weeks. After the cessation of drug therapy enzyme induction may be sustained for about 4 weeks.
Short-term treatment
Women on treatment with enzyme inducing drugs should temporarily use a barrier method or another method of contraception in addition to the CHC. The barrier method must be used during the whole time of the concomitant drug therapy and for 28 days after its discontinuation.
If the drug therapy runs beyond the third patch of an application cycle, the next patch should be started right after the previous one without the usual patch-free interval.
Long-term treatment
In women on long-term treatment with enzyme-inducing active substances, another reliable, nonhormonal, method of contraception is recommended.
The following interactions have been reported in the literature.
Substances increasing the clearance of combined hormonal contraceptives (diminished efficacy of combined hormonal contraceptives by enzyme-induction), e.g.:
Barbiturates, bosentan, carbamazepine, phenytoin, primidone, rifampicin, rifabutin and HIV medication ritonavir, nevirapine and efavirenz and possibly also felbamate, griseofulvin, oxcarbazepine, topiramate, eslicarbazepine, modafinil and products containing the herbal remedy St. John’s Wort (Hypericum perforatum).
Substances with variable effects on the clearance of combined hormonal contraceptives:
When co-administered with combined hormonal contraceptives, many combinations of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, including combinations with HCV inhibitors can increase or decrease plasma concentrations of estrogen or progestins. The net effect of these changes may be clinically relevant in some cases.
Therefore, the prescribing information of concomitant HIV/HCV medications should be consulted to identify potential interactions and any related recommendations. In case of any doubt, an additional barrier contraceptive method should be used by women on protease inhibitor or non-nucleoside reverse transcriptase inhibitor therapy.
Substances increasing drug concentrations of combined hormonal contraceptives (enzyme-inhibitors)
Etoricoxib has been shown to increase plasma concentrations of ethinylestradiol (50 to 60%) when taken concomitantly with an oral triphasic hormonal contraceptive. It is thought that etoricoxib increases ethinylestradiol concentrations because it inhibits sulfotransferase activity thereby inhibiting ethinylestradiol metabolism.
Effects of combined hormonal contraceptives on other medicinal products
Combined hormonal contraceptives may affect the metabolism of certain other active substances. Accordingly, plasma and tissue concentrations may either increase (e.g. ciclosporin) or decrease (e.g. lamotrigine).
Special attention should be paid to the interaction of combined hormonal contraceptives with lamotrigine. Concomitant use is not recommended due to the risk of reduced concentrations and efficacy of lamotrigine (see section 4.4). Initiation of combined hormonal contraception should be avoided during the titration of lamotrigine. In women already treated with lamotrigine, clinical monitoring and dosage adjustment of lamotrigine is required during the initiation of combined hormonal contraceptives and after their discontinuation.
Other forms of interactions
Laboratory tests
The use of contraceptive steroids may influence the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins, e.g. corticosteroid binding globulin and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Changes generally remain within the normal laboratory range.
4.6 Fertility, pregnancy and lactation
Pregnancy
apleek is not indicated during pregnancy. If pregnancy occurs during use of apleek, it must be removed and further use must be stopped. However, extensive epidemiological studies have revealed neither an increased risk of birth defects in children born to women who used combined hormonal contraceptives prior to pregnancy, nor a teratogenic effect when combined hormonal contraceptives were used inadvertently during early pregnancy.
The increased risk of VTE during the postpartum period should be considered when re-starting apleek (see section 4.2 and 4.4).
Breastfeeding
Lactation may be influenced by combined hormonal contraceptives as they may reduce the quantity and change the composition of breast milk. Therefore, the use of combined hormonal contraceptives should generally not be recommended until the nursing mother has completely weaned her child. Small amounts of the contraceptive steroids and/or their metabolites may be excreted with the milk.
Fertility
The use of apleek does not alter the course of future fertility. Upon removal of apleek, women return to their normal fertility.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. No effects on ability to drive and use machines have been observed in users of combined hormonal contraceptives.
4.8 Undesirable effects
Summary of the safety profile
The most commonly reported adverse reactions with Lisvy are application site reactions (rash, pruritus, irritations, erythema, and hypersensitivity). They occur in 20.9 % of users. Rare, serious adverse reactions are arterial and venous thromboembolism.
Tabulated list of adverse reactions
The frequencies of adverse reactions reported in clinical phase 2 and 3 trials with Lisvy (N=3573') are summarised in the table below. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Frequencies are defined as very common (> 1/10), common (> 1/100 to < 1/10), uncommon (>
1/1,000 to < 1/100) and rare (> 1/10,000 to < 1/1,000).
|
System Organ Class (MedDRA) |
Very common |
Common |
Uncommon |
Rare |
|
Psychiatric disorders |
Emotional lability |
Depression/Depressive mood, Decrease and loss of libido | ||
|
Nervous system disorders |
Migraine | |||
|
Vascular disorders |
Venous and arterial thromboembolic events* | |||
|
Gastrointestinal |
Nausea |
'Adverse events in clinical studies were coded using the MedDRA dictionary (version 18.1). The MedDRA preferred term is used to describe a certain reaction and its synonyms and related conditions. Different MedDRA terms representing the same medical phenomenon have been grouped together as single adverse reactions to avoid diluting or obscuring the true effect.
|
System Organ Class (MedDRA) |
Very common |
Common |
Uncommon |
Rare |
|
disorders | ||||
|
Skin and subcutaneous tissue disorders |
Application site reaction | |||
|
Reproductive system and breast disorders |
Genital tract bleeding**, Breast pain |
*- Estimated frequency, from epidemiological studies encompassing a group of combined oral contraceptives. Frequency was borderline to Very Rare.
- ‘Venous and arterial thromboembolic events’ summarizes the following Medical Entities:
Peripheral deep venous occlusion, thrombosis and embolism/Pulmonary vascular occlusion, thrombosis, embolism and infarction/Myocardial infarction/Cerebral infarction and stroke not specified as hemorrhagic
** Contains the Medical Entities Female genital tract bleeding, Unscheduled uterine bleeding
Description of selected adverse reactions
Adverse reactions with very low frequency or with delayed onset of symptoms which are considered to be related to the group of combined hormonal contraceptives, including COCs are listed below (see also sections 4.3 and 4.4):
Circulatory disorders
• An increased risk of arterial and venous thrombotic and thromboembolic events, including myocardial infarction, stroke, transient ischemic attacks, venous thrombosis and pulmonary embolism has been observed in women using CHCs, which are discussed in more detail in section 4.4.
Tumours
• The frequency of diagnosis of breast cancer is very slightly increased among users of combined hormonal contraceptives. As breast cancer is rare in women under 40 years of age the excess number is small in relation to the overall risk of breast cancer. Causation with combined hormonal contraceptive use is unknown.
• Liver tumours (benign and malignant)
Other conditions
• Erythema nodosum, Erythema multiforme
• Women with hypertriglyceridemia (increased risk of pancreatitis when using COCs)
• Hypertension
• Occurrence or deterioration of conditions for which association with COC use is not conclusive: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uremic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosis-related hearing loss
• In women with hereditary angioedema exogenous oestrogens may induce or exacerbate symptoms of angioedema
• Liver function disturbances
• Changes in glucose tolerance or effect on peripheral insulin resistance
• Worsening of Crohn’s disease and of ulcerative colitis
• Worsening of epilepsy
• Chloasma
• Hypersensitivity (including symptoms such as rash, urticaria)
Interactions
Breakthrough bleeding and/or contraceptive failure may result from interactions of other drugs (enzyme inducers) with combined hormonal contraceptives (see section 4.5).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
On the basis of general experience with combined oral contraceptives, symptoms that may possibly occur in this case are: nausea, vomiting and, in young girls, slight vaginal bleeding. There are no antidotes and further treatment should be symptomatic. Additional or improperly used patches should be removed from the skin.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Sex hormones and modulators of the genital system; Progestogens and estrogens, fixed combinations
ATC code: G03AA10 Mechanism of action
The contraceptive effect of combined hormonal contraceptives is based on the interaction of various factors, the most important of which are seen as the inhibition of ovulation and the changes in the cervical secretion.
Clinical efficacy and safety
In the clinical trial performed with Lisvy in the European Union, Latin America and Australia the following Pearl Indices were calculated:
Pearl Index (18-35 years of age, body mass index < 30 kg/m )
Method failure: Pearl Index 0.82 (Upper 95% confidence limit 1.55)
Method and user failure: Pearl Index 1.19 (Upper 95% confidence limit
2.00)
The following Pearl Indices were calculated for the European population:
Pearl Index (18-35 years of age, body mass index < 30 kg/m )
Method failure: Pearl Index 0.40 (Upper 95% confidence limit 1.18)
Method and user failure: Pearl Index 0.76 (Upper 95% confidence limit
1.66)
In the clinical trial performed with Lisvy in the USA the following Pearl Indices were calculated:
Pearl Index (18-35 years of age, no restrictions regarding body mass index) Method failure: Pearl Index 2.91 (Upper 95% confidence limit 4.41)
Method and user failure: Pearl Index 3.92 (Upper 95% confidence limit
5.53)
The failure rate may increase when Lisvy is used incorrectly.
Endometrial histology was investigated in 49 women in one clinical study after 13 cycles of treatment. There were no abnormal results.
5.2 Pharmacokinetic properties
Absorption
After dermal application of apleek, ethinylestradiol and gestodene are well absorbed through the skin. The average release of ethinylestradiol and gestodene over the 7-day application period of apleek results in the same systemic exposure (AUC) during steady-state as observed after daily administration of a combined oral contraceptive containing 0.02 mg ethinylestradiol and 0.06 mg gestodene.
Serum concentrations of ethinylestradiol and gestodene were determined during the third week in different treatment cycles (cycle 1 to cycle 7). Mean maximum serum concentrations of ethinylestradiol in the range of 36-51 ng/L were reached about 1 day after dermal application of the patch. Thereafter, serum concentrations decreased to mean minimum values in the range of 15-23 ng/L at the end of the 1-week application interval. The average concentration during week 3 was in the range of 22-33 ng/L.
Mean maximum serum concentrations of total gestodene in the range of 4.7-7.5 pg/L were reached about 1-1.5 days after dermal application of the patch. Thereafter, serum concentrations decreased to mean minimum values in the range of 2.6-4.0 pg/L at the end of the 1-week application interval. The average concentration during week 3 was in the range of 3.6-5.7 pg/L.
Influence of body weight and body mass index
The serum concentrations of ethinylestradiol and gestodene during treatment with apleek are dependent on the body weight or body mass index of the woman. In obese women with a body mass index > 35 kg/m2, the average serum concentrations of ethinylestradiol and gestodene are 24% and 30% lower, respectively, than those observed in women with a normal body mass index of < 30 kg/m2. Data on contraceptive efficacy in women with Body Mass Index > 30 kg/m2 are limited.
Influence of heat, humidity, exercise and site of application
The pharmacokinetics of ethinylestradiol and gestodene following the application of apleek was investigated under specific conditions of heat, humidity and exercise, i.e., sauna, whirlpool, swimming and different physical exercises in comparison with normal activity. Bioequivalence was generally demonstrated for the parameters Cmax and AUC of ethinylestradiol and gestodene under these specific conditions. The results indicate that there is no clinically relevant difference in the exposure of ethinylestradiol and gestodene observed under specific conditions encountered in a health club such as sauna, whirlpool, swimming or different physical exercises in comparison to normal activities during daily life.
In a formal PK study (non-compartimental analysis) where three different sites of application were tested, mean systemic exposure of gestodene and ethinylestradiol was 24% and 31%, respectively, higher when the patch was applied on the upper outer arm compared to buttocks or abdomen. The range of the exposure data for all three application sites overlapped largely. In a population-PK analysis (metaanalysis), the geometric mean exposure of ethinylestradiol (AUC (0-168) and Cmax) was found to be 41% higher after application to the arm or buttocks in comparison to application to the abdomen. For total gestodene the corresponding differences of mean AUC (0-168) and Cmax values were 26% and 22%, respectively. There is no indication that the mean exposure differences affect the safety or efficacy of apleek.
Comparative data of transdermal apleek to combined oral contraceptive
In a relative bioavailability study, steady-state serum concentrations and pharmacokinetic parameters of ethinylestradiol and gestodene after application of apleek were compared with those of a combined oral contraceptive containing 0.020 mg ethinylestradiol and 0.075 mg gestodene. Mean steady-state Cmax values of ethinylestradiol and gestodene were generally 30%-40% lower after application of apleek compared to the combined oral contraceptive. The exposure (AUC and Cav) of ethinylestradiol was comparable after both routes of administration whereas the exposure based on gestodene (unbound concentration) was 18% lower after application of apleek. These data resulted in average exposure/dose estimates for apleek that are equal to the daily oral administration of 0.020 mg ethinylestradiol and 0.060 mg gestodene. Intersubject variability (%CV) for the major pharmacokinetic
parameters like Cmax and AUC following application of apleek was lower for ethinylestradiol but higher for gestodene relative to those determined after oral administration.
Distribution
Ethinylestradiol is highly but non-specifically bound to serum albumin (approximately 98 %) but not to SHBG. Gestodene is extensively bound to serum albumin and to SHBG. Only about 1 % of the total serum drug concentrations are present as free steroid, 40-80 % is bound to SHBG. Ethinylestradiol induces a strong increase in the serum concentrations of SHBG whereas the administration of gestodene results in a mild decrease of the SHBG concentration. After repeated dermal application of apleek, mean steady-state SHBG serum concentrations are in the range of 201-237 nmol/l.
After intravenous administration of ethinylestradiol, an apparent volume of distribution of 3-9 l/kg was determined. The respective apparent volume of distribution of gestodene is approximately 0.7 l/kg.
Biotransformation
Ethinylestradiol (EE) is primarily metabolized by aromatic hydroxylation but a wide variety of hydroxylated and methylated metabolites are formed, and these are present as free metabolites and as conjugates with glucuronides and sulfate. The major metabolism pathway of ethinylestradiol is the CYP450-dependent 2-hydroxylation and the formation of catechol estrogen 2-hydroxy-EE. The 2-hydroxylation of EE is catalyzed by the CYP2C, CYP2E, and CYP3A gene families. The metabolic clearance rate is in the range of 2-7 ml/min/kg.
Gestodene is completely metabolized to generally more polar metabolites. Metabolism of gestodene is characterized by hydroxylation at several positions of the steroid nucleus and by reduction of the 3-keto function and delta-4 double bond. No active metabolites have been described. Besides CYP3A4, a panel of other Cytochrome P450 enzymes may contribute to a smaller extent to the metabolism of gestodene.
In two studies investigating the effect of CYP3A4 inhibitors (ketoconazole, erythromycin), steady state ethinylestradiol serum concentrations were not influenced by either of the two inhibitors. For gestodene, co-administration of the inhibitors resulted in an 11% and 34% increase of AUC(0-168) for ketoconazole and erythromycin, respectively. This small increase, which results in exposure within the range of marketed combined oral contraceptives, is not considered to be clinically relevant.
In a study investigating the effect of apleek on a single administration of midazolam, a model substrate for substances that are metabolized by CYP3A4, no clinically relevant increase in plasma concentrations of midazolam was observed. Coadministration of midazolam resulted in a minor increase of 7% and 14% for AUC(0-tlast) and Cmax of midazolam, respectively.
Elimination
Ethinylestradiol is not excreted in unchanged form to any significant extent. The metabolites of ethinylestradiol are excreted at a urinary to biliary ratio of 4:6. The serum concentration decline is characterized by at least two disposition phases with a terminal half-life of about 16 hours determined after intravenous administration resulting in generally not quantifiable concentrations two days after patch removal.
Gestodene is not excreted in unchanged form. Its metabolites are excreted at a urinary to biliary ratio of about 6:4. After patch removal, total gestodene serum concentrations decrease more slowly compared to ethinylestradiol with a mean terminal half-life of about 26 hours.
Linearitv/non-linearitv
Pharmacokinetics of ethinylestradiol is linear with regard to dose in the range of 0.020 mg - 0.100 mg. A clinically relevant change in the pharmacokinetics of ethinylestradiol with time has not been observed.
Gestodene pharmacokinetics is dependent on the concentration of SHBG which itself is influenced by oestrogens, androgens and also by gestodene. After repeated dermal application of apleek, 3- to 4-fold elevated SHBG concentrations compared to typical baseline values are observed. Accordingly, steady-state gestodene serum levels differ from those after single application. These SHBG-dependent changes cause a nonlinear change of the pharmacokinetics of gestodene with time. In addition, pharmacokinetics of unbound gestodene was deemed to be concentration-dependent based on three studies investigating the pharmacokinetics of apleek over a period of three cycles. Thus, the pharmacokinetics of gestodene is considered to be non-linear with regard to time and concentration.
Special populations
Gender
apleek is only indicated in women.
Older women
apleek is not indicated after menopause.
Body mass index
Data on contraceptive efficacy in women with Body Mass Index > 30 kg/m2 are limited.
Renal impairment
apleek has not been studied in women with renal impairment. Due to complete metabolization of ethinylestradiol and gestodene to inactive metabolites prior to elimination and due to the availability of a second excretion pathway via the liver no increased risk for women with renal impairment is expected.
Hepatic impairment
apleek has not been studied in women with hepatic impairment. apleek is contraindicated in women with presence or history of severe hepatic disease as long as liver function values have not returned to normal. See also section 4.3.
Ethnic differences
The pharmacokinetics of ethinylestradiol was studied in combination with another progestin in Caucasian, Chinese and Japanese women and did not reveal any clinically significant difference. The pharmacokinetics of apleek has not been specifically studied in women of different ethnicities. No polymorphic enzymes are known that contribute to a major extent in the metabolization of gestodene. The available data in Caucasian, Black and Hispanic women do not indicate any relevant
difference in the pharmacokinetics of apleek between different races/ethnicities. Very limited data is available for Asian women.
Smoking status
There is no indication that smoking has an impact on the pharmacokinetics of ethinylestradiol and gestodene.
Paediatric population
Safety and efficacy have not been established in adolescents under 18 years of age. There is no relevant use of apleek in children and pre-menarchal adolescents.
5.3 Preclinical safety data
Preclinical data on the active ingredients reveal no special risks for humans based on conventional studies of repeated dose toxicity, genotoxicity, local tolerability, carcinogenic potential and toxicity to reproduction. However, it should be borne in mind that sex steroids can promote the growth of certain hormone-dependent tissues and tumours. Biocompatibility studies with the patch and its materials revealed no special risks for humans with regard to the local and systemic safety of the patch.
Environmental Risk Assessment: The active substances gestodene and ethinylestradiol pose a risk to the environment, especially to fish. Moreover, gestodene and ethinylestradiol are persistent in the environment. Any used or unused patches should be discarded according to local requirements. In case of doubt the pharmacist should be consulted (see section 6.6).
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Backing layer
Low density polyethylene (PE) outer layer
Adhesive layer
Adhesive containing:
Ester of Hydrogenated Rosin
Polybutene
Polyisobutylene
Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) Bemotrizinol
Separation foil
Polyethylene terephthalate (PET) film Adhesive matrix
Adhesive containing:
Ester of Hydrogenated Rosin
Polybutene
Polyisobutylene
Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) Release liner
Siliconized polyethylene terephthalate (PET) film
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years
6.4 Special precautions for storage
Store in the original sachet in order to protect from light and moisture. Do not freeze.
6.5 Nature and contents of container
Primary packaging material
A sachet is composed of four layers: a low-density polyethylene film (innermost layer), an aluminium foil, a paper layer and a polyethylene terephthalate film.
A segregation sheet made of siliconized polyethylene terephthalate prevents adhesion of the transdermal patch within the sachet.
Secondary packaging material
Sachets are labelled and packaged together with a booklet (including reminder card and reminder stickers) in a cardboard folding box.
Every folding box contains either 3, 9 or 18 apleek transdermal patches in individual sachets.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
6.6
The patch should be applied immediately upon removal from the protective sachet.
To prevent interference with the adhesive properties of Lisvy, no make-up, creams, lotions, powders or other topical products should be applied to the skin area where Lisvy is or will be placed.
The active substances gestodene and ethinylestradiol pose a risk to the environment, especially to fish. Moreover, gestodene and ethinylestradiol are persistent in the environment. Used patches should not be flushed down the toilet nor placed in liquid waste disposal systems. The used patch should be discarded carefully according to the following instructions.
The original sachet should be kept after use for disposal of the patch. The used patch should be folded in half, adhesive/sticky side inwards. It should be placed in the original sachet and closed by folding the open edge.
There is a two page label on the sachet. The first page of the label should be lifted and be used to seal the folded edge of the sachet. Below the first page, disposal instructions can be found on the second page.
The patch should be discarded safely out of the reach of children or pets.
Any used or unused patches should be discarded according to local requirements. In case of doubt the pharmacist should be consulted.
7 MARKETING AUTHORISATION HOLDER
Gedeon Richter Plc. Gyomroi ut 19-21.
1103 Budapest, Hungary
8 MARKETING AUTHORISATION NUMBER(S)
PL 04854/0145
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
05/03/2014
10 DATE OF REVISION OF THE TEXT
27/06/2016
These incidences were estimated from the totality of the epidemiological study data, using relative risks for the different products compared with levonorgestrel-containing CHCs.
Mid-point of range of 5-7 per 10,000 WY, based on a relative risk for CHCs containing levonorgestrel versus non-use of approximately 2.3 to 3.6.