Metoject Pen 17.5 Mg Solution For Injection In Pre-Filled Pen
Uncommon: may affect up to 1 in 100 people
• Throat inflammation, inflammation of the bowels, vomiting
• Increased sensitivity to light, loss of hair, increased number of rheumatic nodules, shingles, inflammation of blood vessels, herpes-like skin rash, hives
• Onset of diabetes mellitus
• Dizziness, confusion, depression
• Decrease in serum albumin
• Decrease in the number of blood cells and platelets
• Inflammation and ulcer of the urinary bladder or vagina, reduced kidney function, disturbed urination
• Joint pain, muscle pain, osteoporosis (reduction of bone mass)
Rare: may affect up to 1 in 1,000 people
• Increased skin pigmentation, acne, blue spots due to vessel bleeding
• Allergic inflammation of blood vessels, fever, red eyes, infection, wound-healing impairment, decreased number of antibodies in the blood
• Visual disturbances
• Inflammation of the sac around the heart, accumulation of fluid in the sac around the heart
• Low blood pressure, occlusion of a blood vessel by dislodged blood clot (thromboembolic events)
• Lung fibrosis, shortness of breath and bronchial asthma, accumulation of fluid in the sac around the lung
• Electrolyte disturbances
Very rare: may affect up to 1 in 10,000 people
• Profuse bleeding, toxic megacolon (acute toxic dilatation of the gut)
• Increased pigmentation of the nails, inflammation of the cuticles, furunculosis (deep infection of hair follicles), visible enlargement of small blood vessels
• Impaired vision, pain, loss of strength or sensation of numbness or tingling in arms and legs, changes in taste (metallic taste), convulsions, paralysis, severe headache with fever
• Retinopathy (noninflammatory eye disorder)
• Loss of sexual drive, impotence, male breast enlargement (gynaecomastia), defective sperm formation, menstrual disorder, vaginal discharge
• Enlargement of lymphatic nodes (lymphoma)
Subcutaneous application of methotrexate is locally well tolerated. Only mild local skin reactions (such as burning sensations, erythema, swelling, discolouration, severe itching, pain) were observed, decreasing during therapy.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
6. Contents of the pack and other information
What Metoject PEN contains
• The active substance is methotrexate.
1 pre-filled pen with 0.35 ml solution contains 17.5 mg methotrexate.
• The other ingredients are sodium chloride, sodium hydroxide (E 524) and hydrochloric acid (E507) (for pH adjustment) and water for injections.
What Metoject PEN looks like and contents of the pack
Pre-filled pen containing a clear, yellow-brown solution in pre-filled colourless glass syringe with a plunger stopper of rubber and embedded injection needle. The syringe is externally equipped with the device for self-administration. Alcohol pads are included in the package.
Metoject PEN is available in a pack of 1 pre-filled pen.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1DX.
Manufacturer
This product is manufactured by medac Gesellschaft fur klinische Spezialpraparate mbH, Theaterstr. 6, 22880 Wedel, Germany.
| POM | PL: 19488/1800
Leaflet revision date: 22 September 2016
Metoject® is a registered trademark of medac Gesellschaft fur klinische Spezialpraparate mbH.
Metex® is a registered trademark of medac Gesellschaft fur klinische Spezialpraparate mbH.
S1800 LEAFLET Metoject 20160922
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 25°C.
• Keep the pre-filled pen in the outer carton in order to protect from light.
• Do not use this medicine after the expiry date stated on the carton and pre-filled pen after EXP. The expiry date refers to the last day of that month.
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
• If your pre-filled pen shows any signs of deterioration, consult your doctor or pharmacist who will tell you what to do.
S1800 LEAFLET Metoject 20160922
Package leaflet: Information for the user Metoject® PEN 17.5 mg solution for injection in pre-filled
pen
Metex® PEN 17.5 mg solution for injection in pre-filled pen
Methotrexate 17.5 mg solution for injection in pre-filled
pen
(methotrexate)
Your medicine is known as above names but will be referred to as Metoject PEN throughout the following patient information leaflet.
This medicine is also available in other strengths.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist.
This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Metoject PEN is and what it is used for
2. What you need to know before you use Metoject PEN
3. How to use Metoject PEN
4. Possible side effects
5. How to store Metoject PEN
6. Contents of the pack and other information
1. What Metoject PEN is and what it is used for
Metoject PEN is indicated for the treatment of
• active rheumatoid arthritis in adult patients.
• polyarthritic forms of severe, active juvenile idiopathic arthritis, when the response to nonsteroidal anti-inflammatory drugs (NSAIDs) has been inadequate,
• severe recalcitrant disabling psoriasis, which is not adequately responsive to other forms of therapy such as phototherapy, PUVA, and retinoids, and severe psoriatic arthritis in adult patients.
Rheumatoid arthritis (RA) is a chronic collagen disease, characterised by inflammation of the synovial membranes (joint membranes). These membranes produce a fluid which acts as a lubricant for many joints. The inflammation causes thickening of the membrane and swelling of the joint.
Juvenile arthritis concerns children and adolescents less than 16 years. Polyarthritic forms are indicated if 5 or more joints are affected within the first 6 months of the disease.
Psoriasis is a common chronic skin disease, characterised by red patches covered by thick, dry, silvery, adherent scales.
Psoriatic arthritis is a kind of arthritis with psoriatric lesions of the skin and nails, especially at the joints of fingers and toes.
Metoject PEN modifies and slows down the progression of the disease.
2. What you need to know before you use Metoject PEN
Do not use Metoject PEN if you
• are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6).
• suffer from liver or severe kidney diseases or blood diseases.
• regularly drink large amounts of alcohol.
• suffer from a severe infection, such as tuberculosis, HIV or other immunodeficiency syndromes.
• suffer from stomach ulcer or intestinal ulcer.
• are pregnant or breast-feeding.
• receive vaccinations with live vaccines at the same time. Warnings and precautions
Talk to your doctor or pharmacist before taking Metoject PEN if you:
• are elderly or if you feel generally unwell and weak.
• have problems with the way your liver works.
• suffer from dehydration (water loss).
Instructions for use Recommendations
> Carefully read the instructions below before starting your injection.
> Always use the injection technique advised by your doctor, pharmacist or nurse.
Additional information
The manner of handling and throwing away of the medicine and pre-filled pen must be in accordance with local requirements. Pregnant healthcare personnel should not handle and/or administer Metoject PEN.
Methotrexate should not come into contact with the surface of the skin or mucosa. In the event of contamination, the affected area must be rinsed immediately with plenty of water.
What you need in order to administer your injection using the Metoject PEN pre-filled pen You need:
• 1 Metoject PEN pre-filled pen
• 1 alcohol pad
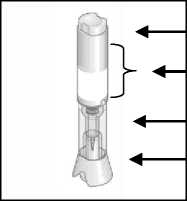
Metoject PEN pre-filled pen components:
■ Injection button Handling area
Transparent control zone Cap
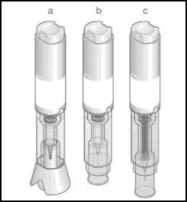
a) With cap before injection
b) After cap removal before injection
c) After injection
What you need to do before administering your injection
1. Wash your hands very carefully.
2. Remove the system from its packaging.
3. Check the Metoject PEN pre-filled pen before using it:
If the Metoject PEN pre-filled pen appears to be damaged do not use it. Use another one and contact your doctor, pharmacist or nurse.
In case a small air bubble is visible through the transparent control zone, this will not affect your dose nor will it harm you.
If you are not able to see or to check the system correctly prior to injection, ask someone around you for assistance.
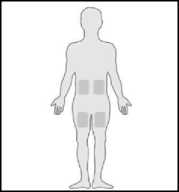
4. Set the Metoject PEN pre-filled pen on a clean flat surface (such as a table). Where you should administer the injection The most appropriate zones for your injection are:
• upper thighs,
• abdomen except around the navel.
• If someone around you administers the injection for you, the person may also use the top of the zone at the back of the arm, just below the shoulder.
Change the injection area with each injection. This will minimize any reactions at the injection site.
Never inject into areas where the skin is tender, bruised, red or hard or where you have scars or stretch marks. If you have psoriasis, you should not try to inject directly into any raised, thick, red or scaly skin patches or lesions.
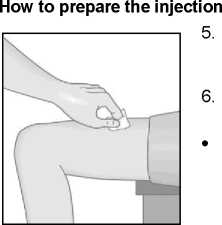
Clean your skin in the chosen injection zone using the enclosed alcohol pad.
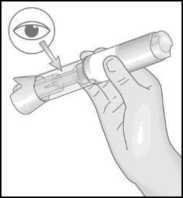
Hold the Metoject PEN pre-filled pen with one hand in the handling area.
Do not remove the cap before you are ready to administer the injection.
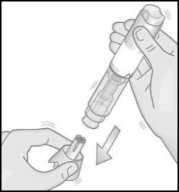
How to administer the injection:
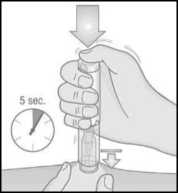
Note:
7. Use your other hand to pull the cap straight off. The small needle shield comes off with the cap automatically. If not, use another pen and contact your doctor, pharmacist or nurse.
• Do not press the button until you are ready to inject.
• If you are unable to remove the cap, ask someone around you for assistance.
Note: Once you have removed the cap, perform your injection without delay.
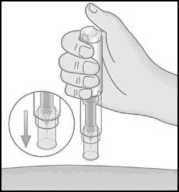
8. With your free hand, build a skin fold by gently squeezing the area of the cleaned skin at the injection site.
• The fold must be held pinched until the Metoject PEN pre-filled pen is removed from the skin after the injection.
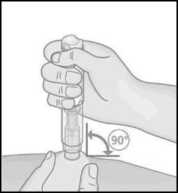
9. Position the uncapped transparent end of Metoject PEN pre-filled pen perpendicular to the fold of skin.
10. Without pressing the button, push the Metoject PEN pre-filled pen firmly onto your skin in order to unlock the button.
• If you are unable to push the Metoject PEN pre-filled pen to the stop-point, ask someone around you for assistance.
11. While holding the Metoject PEN prefilled pen firmly against the skin, now press the button with your thumb.
12. You will hear a click which indicates the start of the injection. Keep holding the pen against the raised skin until all of the medicine is injected. This can take up to 5 seconds.
Do not remove the Metoject PEN pre-filled pen from the skin before the end of the injection to avoid incomplete injection.
If the injection is not triggered, release the button, make sure that the Metoject PEN pre-filled pen is pressed firmly against the skin and push hard on the button.
If you have troubles with your hearing, count 5 seconds from the moment you have pressed the button and then lift the Metoject PEN pre-filled pen from the injection site.
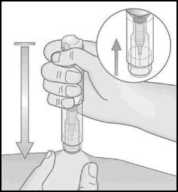
13. Remove the Metoject PEN prefilled pen from the injection site, perpendicular to the skin (pull up).
14. The protective shield automatically moves into place over the needle.
The protective shield is then locked and the needle is protected.
15. In case of a slight bleeding use a plaster.
Before throwing away the Metoject PEN pre-filled pen, check visually that there is no liquid left in the pen, at the bottom of the transparent control zone. If there is liquid left in the pen, not all of the medicine has been injected correctly and you should consult your doctor.
Note
To avoid any injury, never insert your fingers in the opening of
the protective tube covering the needle. Do not destroy the pen.
Whom should you contact in case of need
> For any problem or question, contact your doctor, pharmacist or nurse.
> If you or someone around you is injured by the needle, consult your doctor immediately and throw away the Metoject PEN prefilled pen.
Product Licence holder
Procured from within the EU and repackaged by the Product
Licence holder: S&M Medical Ltd, Chemilines House, Alperton
Lane, Wembley, HAO 1DX.
Recommended follow-up examinations and safety measures:
Even when Metoject PEN is administered in low doses, severe side effects can occur. In order to detect them in time, check-ups and laboratory tests have to be carried out by your doctor.
Before therapy:
Before starting the treatment, blood samples will be taken in order to check that you have enough blood cells, tests to check your liver function, serum albumin (a protein in the blood) and kidney function. Your doctor will also check if you suffer from tuberculosis (infectious disease in combination with little nodules in the affected tissue) and a chest X-ray will be taken.
During therapy:
You will have the following tests at least once a month during the first six months and at least every three months thereafter:
• Examination of the mouth and throat for changes of the mucosa.
• Blood tests.
• Check if your liver is working properly.
• Check if your kidneys are working properly.
• Check of respiratory system and if necessary lung function test.
Methotrexate may affect your immune system and vaccination results. It may also affect the result of immunological tests.
Inactive, chronic infections (such as herpes zoster [shingles], tuberculosis, hepatitis B or C) may flare up. During therapy with Metoject PEN you must not be vaccinated with live vaccines.
Radiation induced dermatitis and sun-burn can reappear under methotrexate therapy (recall-reaction). Psoriatic lesions can exacerbate during UV-irradiation and simultaneous administration of methotrexate.
Enlarged lymph nodes (lymphoma) may occur and if this is the case, therapy must be stopped.
Diarrhoea can be a possible side effect of Metoject PEN and requires an interruption of therapy. If you suffer from diarrhoea please speak to your doctor.
Other medicines and Metoject PEN
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Please note that this also applies to medicines that you will take in the future.
The effect of the treatment may be affected if Metoject PEN is administered at the same time as certain other medicines:
• Medicines harming the liver or the blood count, such as leflunomide.
• Antibiotics (medicines to prevent/fight certain infections) such as: tetracyclines, chloramphenicol, and non-absorbable broad-spectrum antibiotics, penicillines, glycopeptides, sulphonamides (sulphur containing medicines that prevent/ fight certain infections), ciprofloxacin and cefalotin.
• Non-steroidal anti-inflammatory drugs or salicylates
(medicines against pain and/or inflammation).
• Probenecid (medicine against gout).
• Weak organic acids like loop diuretics (“water tablets”) or some medicines used for treatment of pain and inflammatory diseases (such as acetylsalicylic acid, diclofenac and ibuprofen) and pyrazole (e.g. metamizol for treating pain).
• Medicinal products, which may have adverse effects on the bone marrow, such as trimethoprim-sulphamethoxazole (an antibiotic) and pyrimethamine.
• Sulphasalazine (antirheumatic medicine).
• Azathioprine (an immunosuppressive agent sometimes used in severe forms of rheumatoid arthritis).
• Mercaptopurine (a cytostatic agent).
• Retinoids (medicine against psoriasis and other dermatological diseases).
• Theophylline (medicine against bronchial asthma and other lung diseases).
• Proton-pump inhibitors (medicines against stomach trouble).
• Hypoglycaemics (medicines that are used to lower the blood sugar).
Vitamins containing folic acid may impair the effect of your treatment and should only be taken when advised by your doctor.
Vaccination with live vaccine must be avoided.
Metoject PEN with food, drink and alcohol
Alcohol as well as large amounts of coffee, caffeine-containing soft drinks and black tea should be avoided during treatment with Metoject PEN.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Do not take Metoject PEN during pregnancy. Men and women should use an effective method of birth control during treatment and during a further six months after treatment with Metoject PEN has been discontinued.
In women of child-bearing age, any existing pregnancy must be excluded with certainty by taking appropriate measures, such as pregnancy test, prior to therapy.
As methotrexate can be genotoxic, all women who wish to become pregnant are advised to consult a genetic counselling centre, if possible, already prior to therapy. Men should seek advice about the possibility of sperm preservation before starting therapy.
Stop breast-feeding prior to and during treatment with Metoject PEN.
Driving and using machines
Treatment with Metoject PEN may cause adverse reactions affecting the central nervous system, such as tiredness and dizziness. Thus the ability to drive a vehicle and/or to operate machines may, in certain cases, be compromised. If you feel tired or drowsy do not drive or use machines.
Metoject PEN contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per dose; i.e. essentially “sodium-free”.
3. How to use Metoject PEN
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor decides on the dosage, which is adjusted individually to you. Usually it takes 4-8 weeks before there is any effect of the treatment.
Metoject PEN is administered subcutaneously (under the skin) by or under the supervision of a physician or healthcare staff as an injection once a week only. Together with your doctor you decide on a suitable weekday each week on which you receive your injection.
Use in children and adolescents
The doctor decides on the appropriate dose in children and adolescents with polyarthritic forms of juvenile idiopathic arthritis. Metoject PEN is not recommended in children less than 3 years of age due to insufficient experience in this age group.
Method and duration of administration
Metoject PEN is injected once weekly!
The duration of the treatment is determined by the treating physician. Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris and psoriatic arthritis with Metoject PEN is a long-term treatment.
At the start of your therapy, Metoject PEN will be injected by medical staff. However, your doctor may decide that you are able to learn how to inject Metoject PEN under the skin yourself. You will then receive appropriate training.
Under no circumstances should you try to inject Metoject PEN yourself before you have received such training.
You can also find guidance on how to use Metoject PEN in the section “Instructions for use” at the end of this leaflet. Please note that all of the contents have to be used.
The manner of handling and throwing away of the medicine and pre-filled pen must be in accordance with local requirements. Pregnant health care personnel should not handle and/or administer Metoject PEN.
Methotrexate should not come into contact with the surface of the skin or mucosa. In the event of contamination, the affected area must be rinsed immediately with plenty of water.
If you use more Metoject PEN than you should
If you use more Metoject PEN than you should, talk to your doctor immediately.
If you forget to use Metoject PEN
Do not take a double dose to make up for a forgotten dose.
If you stop using Metoject PEN
If you stop using Metoject PEN, talk to your doctor immediately.
If you have the impression that the effect of Metoject PEN is too strong or too weak, talk to your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency as well as the degree of severity of the side effects depends on the dosage level and the frequency of administration.
As severe side effects may occur even at low dosage, it is important that you are monitored regularly by your doctor. Your doctor will do tests to check for abnormalities developing in the blood (such as low white blood cells, low platelets, lymphoma) and changes in the kidneys and the liver.
Tell your doctor immediately if you experience any of the
following symptoms, as these may indicate a serious, potentially life-threatening side effect, which require urgent specific treatment:
• persistent dry, non-productive cough, shortness of breath and fever; these may be signs of an inflammation of the lungs (pneumonia) [common - may affect up to 1 in 10 people]
• symptoms of liver damage such as yellowing of the skin and whites of the eyes; methotrexate can cause chronic liver damage (liver cirrhosis), formation of scar tissue of the liver (liver fibrosis), fatty degeneration of the liver [all uncommon -may affect up to 1 in 100 people], inflammation of the liver (acute hepatitis) [rare - may affect up to 1 in 1,000 people] and liver failure [very rare - may affect up to 1 in 10,000 people]
• allergy symptoms such as skin rash including red itchy skin, swelling of the hands, feet, ankles, face, lips, mouth or throat (which may cause difficulty in swallowing or breathing) and feeling you are going to faint; these may be signs of severe allergic reactions or an anaphylactic shock [rare - may affect up to 1 in 1,000 people]
• symptoms of kidney damage such as swelling of the hands, ankles or feet or changes in frequency of urination or decrease or absence of urine; these may be signs of kidney failure [rare - may affect up to 1 in 1,000 people]
• symptoms of infections, e.g. fever, chills, achiness, sore throat; methotrexate can make you more susceptible to infections. Rarely [may affect up to 1 in 1,000 people] severe infections like a certain type of pneumonia (Pneumocystis carinii pneumonia) or blood poisoning (sepsis) may occur
• severe diarrhoea, vomiting blood and black or tarry stools;
these symptoms may indicate a rare [may affect up to 1 in 1,000 people] severe complication of the gastrointestinal system caused by methotrexate e.g. gastrointestinal ulcers
• fever and serious deterioration of your general condition, or sudden fever accompanied by a sore throat or mouth, or urinary problems; methotrexate can very rarely [may affect up to 1 in 10,000 people] cause a sharp fall in white blood cells (agranulocytosis) and severe bone marrow suppression
• unexpected bleeding, e.g. bleeding gums, blood in the urine, vomiting blood or bruising, these can be signs of a severely reduced number of blood platelets caused by severe courses of bone marrow depression [very rare - may affect up to 1 in 10,000 people]
• severe skin rash or blistering of the skin (this can also affect your mouth, eyes and genitals); these may be signs of the very rare [may affect up to 1 in 10,000 people] conditions called Stevens Johnson syndrome or burned skin syndrome (toxic epidermal necrolysis)
In the following, please find the other side effects that may occur: Very common: may affect more than 1 in 10 people
• Mouth inflammation, indigestion, nausea (feeling sick), loss of appetite
• Increase in liver enzymes.
Common: may affect up to 1 in 10 people
• Mouth ulcers, diarrhoea
• Rash, reddening of the skin, itching
• Headache, tiredness, drowsiness
• Reduced blood cell formation with decrease in white and/or red blood cells and/or platelets (leukopenia, anaemia, thrombocytopenia)