Mictonorm 15 Mg Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Mictonorm 15 mg Coated Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each coated tablet contains 15 mg propiverine hydrochloride equivalent to 13.64 mg propiverine.
Each coated tablet contains 63 mg lactose monohydrate, 0.6 mg glucose monohydrate, 49 mg sucrose, and 0.15 mg Cochineal Red A (E 124).
For full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Coated tablets
Rose-coloured, biconvex, round sugar-coated tablets
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Symptomatic treatment of urinary incontinence and/or increased urinary frequency and urgency as may occur in patients with overactive bladder syndrome or neurogenic detrusor overactivity (detrusor hyperreflexia) from spinal cord injuries, e.g. transverse lesion paraplegia.
4.2 Posology and method of administration
Coated tablets for oral application
The recommended daily doses are as follows:
Adults: As a standard dose one coated tablet (= 15 mg propiverine hydrochloride) twice a day is recommended, this may be increased to three times a day. Some patients may already respond to a dosage of 15 mg a day.
For neurogenic detrusor overactivity a dose of one coated tablet three times a day is recommended. The maximum recommended daily dose is 45 mg.
Elderly: Generally there is no special dosage regimen for the elderly (see 5.2).
Use in renal impairment
In patients with mild to moderate impaired renal function there is no need for a dose adjustment. In the treatment of this group of patients caution has to be exercised. In patients with severe renal impairment (creatinine clearance < 30 ml/min) the maximum daily dose is 30 mg.
Use in hepatic impairment
In patients with mild impaired hepatic function there is no need for a dose adjustment but caution should be exercised. The treatment of patients with moderate to severe impairment is not recommended because no data are available.
A high fat meal increases the bioavailability of propiverine. Therefore, propiverine should be taken before a meal, especially in patients with renal or hepatic impairment (see 5.2).
Patients receiving concomitant treatment with drugs that are potent inhibitors of CYP 3A4 combined with methimazole
In patients receiving drugs that are potent FMO inhibitors such as methimazole in combination with potent CYP 3A4/5 inhibitors treatment should start with a dose of 15 mg per day. The dose may be titrated to a higher dose. However, caution should be exercised and clinicians should monitor these patients carefully for side effects (see 4.4, 4.5, 5.2).
This medicinal product contains 0.61 mg of glucose. Accordingly, a daily dose of 2 coated tablets supplies 1.22 mg of glucose.
4.3 Contraindications
The drug is contraindicated in patients who have demonstrated hypersensitivity to the active substance or to any of the excipients and in patients suffering from one of the following disorders: obstruction of the bowel
significant degree of bladder outflow obstruction where urinary retention may be
anticipated
myasthenia gravis
intestinal atony
severe ulcerative colitis
toxic megacolon
uncontrolled angle closure glaucoma moderate or severe hepatic impairment tachyarrhythmias.
4.4 Special warnings and precautions for use
The drug should be used with caution in patients suffering from:
- autonomic neuropathy
- renal impairment
- hepatic impairment
Symptoms of the following diseases may be aggravated following administration of the drug:
- severe congestive heart failure (NYHA IV)
- prostatic hypertrophy
- hiatus hernia with reflux oesophagitis
- cardiac arrhythmia
- tachycardia
Propiverine, like other anticholinergics, induces mydriasis. Therefore, the risk to induce acute angle-closure glaucoma in individuals predisposed with narrow angles of the anterior chamber may be increased.
Drugs of this class have been reported to induce or precipitate acute angle-closure glaucoma.
Pollakiuria and nocturia due to renal disease or congestive heart failure as well as organic bladder diseases (e.g. urinary tract infections, malignancy) should be ruled out prior to treatment.
In patients receiving drugs that are potent FMO inhibitors such as methimazole in combination with potent CYP 3A4/5 inhibitors treatment should start with a dose of 15 mg per day. The dose may be titrated to a higher dose. However, caution should be exercised (see 4.2, 4.5, 5.2).
This product contains lactose monohydrate, glucose monohydrate and sucrose.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency, fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medication.
Cochineal red A (E124, lake) may cause allergic reactions.
Due to a lack of data Mictonorm 15 mg Coated Tablets should not be used in children.
4.5 Interaction with other medicinal products and other forms of interaction
Increased effects due to concomitant medication with tricyclic antidepressants (e. g. imipramine), tranquillisers (e.g. benzodiazepines), anticholinergics, amantadine, neuroleptics (e. g. phenothiazines) and beta-adrenoceptor agonists (beta-sympathomimetics). Decreased effects due to concomitant medication with cholinergic drugs. Reduced blood pressure in patients treated with isoniazid. The effect of prokinetics such as metoclopramide may be decreased.
Pharmacokinetic interactions are possible with other drugs metabolised by cytochrome P450 3A4 (CYP 3A4). However, a very pronounced increase of concentrations for such drugs is not expected as the effects of propiverine are small compared to classical enzyme inhibitors (e.g. ketoconazole or grapefruit juice). Propiverine may be considered as weak inhibitor of cytochrome P450 3A4. Pharmacokinetic studies with patients concomitantly receiving potent CYP 3A4 inhibitors such as azole antifungals (e.g. ketoconazole, itraconazole) or macrolide antibiotics (e.g. erythromycin, clarithromycin) have not been performed.
In patients receiving drugs that are potent FMO inhibitors such as methimazole in combination with potent CYP 3A4/5 inhibitors treatment should start with a dose of 15 mg per day. The dose may be titrated to a higher dose. However, caution should be exercised (see 4.2, 5.2).
4.6 Pregnancy and lactation
In animal studies, skeletal retardation in the offspring occurred when the drug was administered orally at high doses to pregnant females. The drug was also secreted into the milk of lactating mammals.
Propiverine hydrochloride should therefore not be administered to pregnant or nursing women.
4.7 Effects on ability to drive and use machines
Propiverine hydrochloride may produce drowsiness and blurred vision. This may impair the patient's ability to exert activities that require mental alertness such as operating a motor vehicle or other machinery, or to exert hazardous work while taking this drug.
Sedative drugs may enhance the drowsiness caused by propiverine hydrochloride
4.8 Undesirable effects
|
Adverse reactions |
System organ class (Disorders according to MedDRA) |
|
Very common (>1/10) dry mouth |
Gastrointestinal |
|
Common(>1/100, <1/10) accommodation abnormal, accommodation disturbances, vision abnormal |
Eye |
|
fatigue |
General disorders and administration site conditions |
|
headache |
Body as a whole - general disorders |
|
abdominal pain, dyspepsia |
Gastro-intestinal |
|
constipation |
Gastrointestinal |
|
Uncommon (>1/1,000, <1/100) | |
|
nausea/vomiting |
Gastrointestinal |
|
dizziness |
Nervous system |
|
tremor |
Nervous system |
|
urinary retention |
Urinary system |
|
flushing |
Vascular |
|
dysgeusia |
Special senses other disorders |
|
decreased blood pressure with drowsiness |
Vascular |
|
Rare (>1/10,000, <1/1,000) | |
|
rash due to idiosyncrasy (propiverine hydrochloride) or hypersensitivity (excipients, e. g. colorant) |
Skin and subcutaneous tissue |
|
Very rare (<1/10,000, including isolated reports) | |
|
palpitation |
Cardiac |
|
restlessness, confusion |
Psychiatric |
|
Not known (cannot be estimated from the available data) | |
|
hallucination |
Psychiatric |
All undesirable effects are transient and recede after a dose reduction or termination of the therapy after maximum 1 - 4 days.
During long-term therapy hepatic enzymes should be monitored, because reversible changes of liver enzymes might occur in rare cases. Monitoring of intraocular pressure is recommended in patients at risk of developing glaucoma.
Particular attention should be paid to the residual urine volume in cases of urinary tract infection.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Overdose with the muscarinic receptor antagonist propiverine hydrochloride can potentially result in central anticholinergic effects, e.g. restlessness, dizziness, vertigo, disorders in speech and vision and muscular weakness. Moreover, severe dryness of mucosa, tachycardia and urinary retention may occur.
Treatment should be symptomatic and supportive. Management of overdose may include initiation of vomiting or gastric lavage using an oiled tube (attention: dryness of mucosa!), followed by symptomatic and supportive treatment as for atropine overdose (e.g. physostigmine) with a dosage of 1.0 to 2.0 mg in adults by slow intravenous injection (may be repeated as necessary to a total of 5 mg).
A 14-year old girl who ingested 450 mg propiverine hydrochloride presented with confabulation. The adolescent fully recovered.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC code: G04BD06
Pharmacotherapeutic group: spasmolytic, anticholinergic
Mechanism of action
Inhibition of calcium influx and modulation of intracellular calcium in urinary bladder smooth muscle cells causing musculotropic spasmolysis.
Inhibition of the efferent connection of the nervus pelvicus due to anticholinergic action.
Pharmacodynamic effects
In animal models propiverine hydrochloride causes a dose-dependent decrease of the intravesical pressure and an increase in bladder capacity.
The effect is based on the sum of the pharmacological properties of propiverine and the active urinary metabolites as shown in isolated detrusor strips of human and animal origin.
5.2 Pharmacokinetic properties
General characteristics of the active substance
Propiverine is nearly completely absorbed from the gastrointestinal tract. It undergoes extensive first pass metabolism. Effects on urinary bladder smooth muscle cells are due to the parent compound and three active metabolites as well, which are rapidly excreted into the urine.
Absorption
After oral administration of Mictonorm 15 mg Coated Tablets propiverine is rapidly absorbed from the gastrointestinal tract with maximal plasma concentrations reached after 2.3 hours. The mean absolute bioavailability of Mictonorm 15 mg Coated Tablets is 40.5 % (arithmetic mean value for AUC0-0O (p.o.) / AUC0-0O (i.v.)).
Food intake increases the bioavailability of propiverine (mean increase 1.3fold), but does not significantly affect the maximum plasma concentrations of propiverine or of its main metabolite, propiverine-N-oxide. This difference in bioavailability is unlikely to be of clinical significance but adjustment of dose in relation to food intake could be required in patients suffering from impaired renal or hepatic function. Therefore, a regular intake before meals is recommended.
Distribution
After administration of Mictonorm 15 mg Coated Tablets t.i.d., steady state is reached after four to five days at a higher concentration level than after single dose application (Caverage = 61 ng/ml). The volume of distribution was estimated in 21 healthy volunteers after intravenous administration of propiverine hydrochloride to range from 125 to 473 l (mean 279 l) indicating, that a large amount of available propiverine is distributed to peripheral compartments. The binding to plasma proteins is 90 - 95 % for the parent compound and about 60 % for the main metabolite.
Plasma concentrations of propiverine in 16 healthy volunteers after single and repeated administration of Mictonorm 15 mg Coated Tablets (t.i.d. for 6 days):
single dose multiple dose
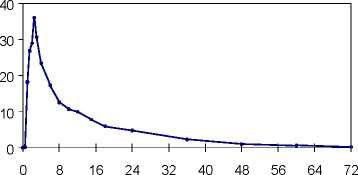
time [h]
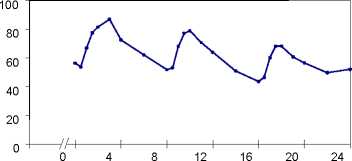
time [h]
Steady state characteristics of propiverine following multiple-dose administration to 16 healthy volunteers of Mictonorm 15 mg Coated Tablets (t.i.d. for 6 days):
|
Dose interval |
AUC0_t |
PTF |
C average | |||
|
[h] |
[ngh/ml] |
CV [%] |
[%] |
CV [%] |
[ng/ml] |
CV [%] |
|
0 - 8 |
515 |
35 |
57 |
16 |
64 |
36 |
|
8 - 16 |
460 |
33 |
70 |
25 |
57 |
33 |
|
16 - 24 |
421 |
36 |
52 |
39 |
52 |
36 |
|
CV: coefficient of variation | ||||||
|
PTF: peak-trough fluctuation | ||||||
Biotransformation
Propiverine is extensively metabolised by intestinal and hepatic enzymes. The primary metabolic route involves the oxidation of the Piperidyl-N and is mediated by CYP 3A4 and Flavin-monoxygenases (FMO) 1 and 3 and leads to the formation of the much less active N-oxide, the plasma concentration of which greatly exceeds that of the parent substance. Four metabolites were identified in urine; two of them are pharmacologically active and may contribute to the therapeutic efficacy of Mictonorm 15 mg Coated Tablets.
In vitro there is a slight inhibition of CYP 3A4 and CYP 2D6 detectable which occurs at concentrations exceeding therapeutic plasma concentrations 10- to 100-fold (see section 4.5).
Elimination
Following administration of 30 mg oral dose of 14C-propiverine hydrochloride to healthy volunteers, 60 % of radioactivity was recovered in urine and 21 % was recovered in faeces within 12 days. Less than 1% of an oral dose is excreted unchanged in the urine. Mean total clearance after single dose administration of 30 mg is 371 ml/min (191 - 870 ml/min). In three studies including a total of 37 healthy volunteers the mean elimination half-life was 14.1, 20.1, and 22.1 hours, respectively.
Linearity/ non-linearity
Pharmacokinetic parameters of propiverine and propiverine-N-oxide following oral administration of 10 - 30 mg of propiverine hydrochloride are linearly related to dose.
There are no changes of pharmacokinetics during steady state compared to single dose administration.
Characteristics in patients
Renal impairment:
Severe renal impairment does not significantly alter the disposition of propiverine and its main metabolite, propiverine-N-oxide, as deduced from a single dose study in 12 patients with creatinine clearance < 30 ml/min. No dose adjustment is to be recommended as long as the total daily dose does not exceed 30 mg (i.e. Mictonorm 15 mg Coated Tablets given b.i.d.). In case that higher dose (i.e. 45 mg) shall be administered a careful titration of dose is recommended considering anticholinergic effects as a marker for tolerability.
Hepatic insufficiency:
There were similar steady state pharmacokinetics in 12 patients with mild to moderate impairment of liver function due to fatty liver disease as compared to 12 healthy controls. No data are available for severe hepatic impairment.
Age:
The comparison of trough plasma concentrations during steady state (Mictonorm 15 mg Coated Tablets t.i.d. for 28 days) reveals no difference between older patients (60 - 85 years; mean 68) and young healthy subjects. The ratio of parent drug to metabolite remains unchanged in older patients indicating the metabolic conversion of propiverine to its main metabolite, propiverine-N-oxide, not to be an age-related or limiting step in the overall excretion.
Patients with glaucoma:
Intraocular pressure in patients with open angle glaucoma and in patients with treated (controlled) angle closure glaucoma is not increased by Mictonorm 15 mg Coated Tablets t.i.d., as demonstrated by two placebo-controlled studies.
5.3 Preclinical safety data
In long term oral dose studies in two mammalian species the main treatment related effects were changes in the liver (including elevation of hepatic enzymes). These were characterised by hepatic hypertrophy and fatty degeneration. The fatty degeneration was reversible upon cessation of treatment.
In animal studies, skeletal retardation in the offspring occurred when the drug was administered orally at high doses to pregnant females. In lactating mammals propiverine hydrochloride was excreted into the milk.
There was no evidence of mutagenicity. The carcinogenicity study in mice demonstrated an increased incidence of hepatocellular adenoma and carcinoma in high dose males. In the rat carcinogenicity study hepatocellular adenoma, kidney adenoma and urinary bladder papilloma has been demonstrated in high dose male rats, while in female animals endometrial stromal polyps were increased at the high dose levels. Both the rat and mouse tumours were considered to be species specific and therefore not of clinical relevance.
6.1 List of excipients
Tablet core:
Lactose monohydrate, powdered cellulose, magnesium stearate,
Tablet coat:
sucrose
talc
heavy kaolin calcium carbonate titanium dioxide (E171) acacia gum
colloidal anhydrous silica Macrogol 6000 glucose monohydrate Cochineal red A (E124, lake) montan wax.
6.2 Incompatibilities
Not applicable
6.3 Shelf life
3 years
6.4 Special precautions for storage
No special precautions for storage
6.5 Nature and contents of container
PVC/aluminium blisters are available in cartons of 14, 20, 28, 30, 50, 56, 60, 84, 100, 112, 168, 252 or 300 coated tablets.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements
7 MARKETING AUTHORISATION HOLDER
APOGEPHA Arzneimittel GmbH KyffhauserstraBe 27 01309 Dresden Germany
8 MARKETING AUTHORISATION NUMBER(S)
PL 15072/0002
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
23/04/1998 / 23/04/2008
10 DATE OF REVISION OF THE TEXT
28/10/2014