Minims Phenylephrine Hydrochloride 2.5%W/V
|
Graphics&Packing __ _ _ _ _ _ _ __ Technology Section ULOTKA PIL RAUSCH 1 LOMR ICN POLFA RZESZOW S.A. H ■ ■ mm | |||||
|
Nazwa produktu Product Name |
Minims Phenylephrine Hydrochloride 10% (and 2,5%) w/v DRP 20 SDU |
Kolor nadruku Colours | |||
|
Black | |||||
|
Kraj Country (ISO) |
United Kingdom (UK), Ireland (IE) |
Nr wykrojnika Spec No |
5338 | ||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
76922 | |||
|
Nr korekty Proof No |
6 °a*a 03.06.2016 Date |
Kod farmaceutyczny Pharmacode |
- | ||
|
Kod wersji Valeant version code |
P1UKIE03 |
Inny kod Other code |
76922 (Data Matrix) | ||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
160 x 240 mm | ||
|
Kroj czcionki Font used |
Frutiger Neue LT W1G Condensed Frutiger Neue LT W1G Condensed Bold |
Gramatura papieru Paper weight |
60 g/m2 | ||
|
Komentarze Comments (Reason for the change) | |||||
|
PRAC (Safety change) update PMRJCN-15-1294-A Packing Site: Laboratoire Chauvin, Aubenas, France | |||||
|
Akceptacja Techniczna Technical Approval | |||||
|
Akceptacja Dziafu ds. Rejestracji Regulatory Affairs Dept. Approval | |||||
76922
Art.76922 P1UKIE03

Package leaflet: Information for the user
Minims® Phenylephrine Hydrochloride 2.5% w/v Minims® Phenylephrine Hydrochloride 10% w/v
Eye Drops, solution Phenylephrine Hydrochloride
Read all of this leaflet carefully before you start using this medicine because It contains Important Information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4._
What is in this leaflet:
1. What Minims® Phenylephrine Hydrochloride eye drops are and what they are used for
2. What you need to know before you use Minims® Phenylephrine Hydrochloride eye drops
3. Howto use Minims® Phenylephrine Hydrochloride eye drops
4. Possible side effects
5. How to store Minims® Phenylephrine Hydrochloride eye drops
6. Contents of the pack and other information
1. What Minims ' Phenylephrine Hydrochloride eye drops are and what are they used for
Minims® Phenylephrine Hydrochloride contains a mydriatic agent. It is used to enlarge the pupil of your eye. Your doctor or eye specialist may use it to examine your eye more closely or as part of a treatment program.
Minims® Phenylephrine Hydrochloride 2.5% is indicated for children, adults and the elderly.
Minims® Phenylephrine Hydrochloride 10% is not indicated for children or the elderly, as this concentration is too strong. The doctor or eye specialist will use an alternative medicine in these patients.
2. What you need to know before you use Minims' Phenylephrine Hydrochloride eye drops
Do not use Minims® Phenylephrine Hydrochloride drops if:
• you are allergic to the active substance (phenylephrine) or any of the other ingredients of this medicine (listed in section 6)
• you have a heart disease
• you have fast heartbeats (tachycardia)
• you have high blood pressure
• you have bulges in major blood vessels (aneurysms)
• you have an overactive thyroid gland (hyperthyroidism)
• you have high blood sugar level(diabetes mellitus)
• Patients on monoamine oxidase inhibitors, tricyclic antidepressants and anti-hypertensive agents (including beta-blockers)
• Patients with closed angle glaucoma (unless previously treated with iridectomy) and patients with a narrow angle prone to glaucoma precipitated by mydriatics
• Use in patients wearing contact lenses.
Warnings and precautions
Use with caution in children and the elderly.
Ask yourself the following questions:
• Do you have narrow arteries in the head (cerebral arteriosclerosis)?
• Do you have asthma?
• Do your eyes develop increased internal pressure when the pupil is enlarged? The doctor or eye specialist will have checked to make sure that this problem will not affect you.
• Are your eyes red or inflamed?
If the answer to any of these questions is yes, tell your doctor or eye specialist.
The active substance can cause the cornea to become cloudy if the surface of the cornea is damaged. The cornea is the transparent membrane covering the surface of the eye. Your doctor or eye specialist will take this into account before deciding to give you these eye drops.
Other medicines and Minims® Phenylephrine Hydrochloride
Tell your doctor or eye specialist if you are taking, nave recently taken or might take any other medicines.
• Medicines for depression
• Any medicines to reduce your blood pressure (including medicines called betablockers)
• Medicines to treat irregular heartbeats (quinidine or medicines called cardiac glycosides)
The active substance can react with several types of anaesthetic (e.g. halothane). Your doctor will know about this. If you are having an operation your doctor will make sure that this problem does not affect you.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or eye specialist for advice before taking this medicine as he/ she may decide to use these drops or to prescribe you an alternative.
Contact lenses
Your doctor will ask you to remove your contact lenses prior to the administration of the drops. There is no information on the effect of this product on contact lenses. Therefore, do not wear your contact lenses until the effects of the drops have completely worn off.
Driving and using machines
Some people find bright light (such as daylight) uncomfortable for a few hours after receiving Minims® Phenylephrine Hydrochloride.
This may make it difficult for you to drive. As with all eye drops, your vision may be blurred after putting the drops in. Wait until your vision is clear before driving or using machines.
3. Howto use Minims' Phenylephrine Hydrochloride eye drops
Sometimes a drop of local anaesthetic may be used prior to Minims® Phenylephrine Hydrochloride to prevent the stinging effect of phenylephrine eye drops. The drops are for single use only and provided in single use Minims units, to treat both eyes if both eyes are affected. Once opened, use immediately. Discard any unused portion.
Dosage
The recommended dose is one drop in each eye. If necessary this can be repeated once only at least one hour after the first drop.
2016-06-03 10:57:13
PlUKIE03_76922_Minims-Phenylephrine_Hyd.rochloride_2-5_10_WV_DRP_20SDU_UK_IE_030616_06.indd 1
|
Graphics&Packing __ _ _ _ _ _ _ __ Technology Section ULOTKA PIL RAUSCH 1 LOMR ICN POLFA RZESZOW S.A. H ■ ■ mm | |||||
|
Nazwa produktu Product Name |
Minims Phenylephrine Hydrochloride 10% (and 2,5%) w/v DRP 20 SDU |
Kolor nadruku Colours | |||
|
Black | |||||
|
Kraj Country (ISO) |
United Kingdom (UK), Ireland (IE) |
Nr wykrojnika Spec No |
5338 | ||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
76922 | |||
|
Nr korekty Proof No |
6 °a*a 03.06.2016 Date |
Kod farmaceutyczny Pharmacode |
- | ||
|
Kod wersji Valeant version code |
P1UKIE03 |
Inny kod Other code |
76922 (Data Matrix) | ||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
160 x 240 mm | ||
|
Kroj czcionki Font used |
Frutiger Neue LT W1G Condensed Frutiger Neue LT W1G Condensed Bold |
Gramatura papieru Paper weight |
60 g/m2 | ||
|
Komentarze Comments (Reason for the change) | |||||
|
PRAC (Safety change) update PMRJCN-15-1294-A Packing Site: Laboratoire Chauvin, Aubenas, France | |||||
|
Akceptacja Techniczna Technical Approval | |||||
|
Akceptacja Dziafu ds. Rejestracji Regulatory Affairs Dept. Approval | |||||
Art.76922 P1UKIE03
Phenylephrine 2.5% w/v: For examination of the eye, the normal dose for adults, elderly and children, is one drop in each eye. If necessary this can be repeated once only at least one hour after the first drop.
Phenylephrine 10% w/v: For adults the same dose is recommended. Flowever, Minims Phenylephrine 10% w/v is not recommended for use in children or elderly patients.If you are receiving this product as part of a treatment programme your doctor may suggest an additional dose schedule of one drop every 24 hours of the 2.5% w/v product.
Method of administration
Parents must be careful not to get this preparation in their children's mouth or cheeks and to wash their hands and the child's hands or cheeks following administration.
The doctor or eye specialist will normally put the drops in your eye for you, but if you are putting the eye drops in yourself, then follow these instructions carefully:
1. Wash your hands.
2. Use a mirror, this will help you to see what you are doing.
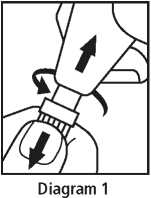
3. Carefully dry your eyes using a tissue. Twist and pull cap to separate from the Minims body (Diagram 1).
4. Pull your lower eyelid down gently, and then carefully place 1 drop inside your lower eyelid.
5. Close your eye and blink a few times to make sure your eye is covered by the liquid.
6. Close the eyelids. It is advisable to press gently on the inner corner of your eyelid for 3 minutes. This will help stop the solution draining into your nose and throat.
7. Repeat steps 4, 5 and 6 for the other eye if necessary, then throw away the Minims unit, even if some solution remains.
If you use more Minims1 2 Phenylephrine Hydrochloride than you should, an overdose is very unlikely to occur. If you suddenly feel unwell after receiving the drops, tell your doctor or eye specialist or contact your nearest emergency department immediately.
If you forget to use Minims2 Phenylephrine Hydrochloride, you should take the missed dose as soon as you remember, unless it is nearly time for the next dose, but you should not take a double dose to make up fora missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Minims® Phenylephrine Hydrochloride may sting when the drops are first put in and your vision may become blurred. This will gradually wear off. Serious side effects
• Rarely, more serious reactions, including spasm of the artery of the heart, heart irregularities and heart attacks, have been reported after using phenylephrine solutions. These have usually occurred in patients using some 10 % phenylephrine and already suffering from heart disease.
Other side effects
Not known: frequency cannot be estimated from the available data.
• In some cases bright light can be uncomfortable for a few hours after receiving the drops.
• Occasionally allergic reactions or conjunctival sensitivity (abnormal sensitivity of the conjunctiva (thin, clear, moist membrane that coats the white outer surface of the eye) to various substances with discomfort upon instillation) can occur.
• The active substance can sometimes affect your circulatory system, causing increased blood pressure and palpitations (a sensation in which a person is aware of an irregular, hard, or rapid heartbeat), tachycardia (fast heartbeats) and other heart irregularities (extrasystoles and cardiac arrythmias).
• Paleness around the eyes in preborn babies (this is relevant to the 2.5% formulation).
Additional side effects in children:
Not known: frequency cannot be estimated from the available data
• Fluid or swelling in the lungs (this is relevant to the 10% formulation).
Reporting of side effects
If you or your child get any side effects, talk to your doctor, the doctor treating your child or pharmacist. This includes any side effects not listed in this leaflet.
You can also report side effects directly via:
United Kingdom
The Yellow Card Scheme at: www.mhra.gov.uk/yellowcard Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, IRL- Dublin 2;
Tel: +353 1 6764971; Fax: +353 1 6762517; Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store your eye drops
Keep this medicine out of the sight and reach of children.
Do not use the eye drops after the expiry date which is stated on the overwrap of each unit and on the carton after the letters "EXP".
The expiry date refers to the last day of that month.
Do not store above 25°C. Do not freeze. Store in the original package in order to protect from light.
The drops are for single use only and provided in single use Minims units, to treat both eyes if both eyes are affected. Once opened, use immediately. Discard any unused portion. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist howto dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
2016-06-03 10:57:13
What do your eye drops contain
• The active ingredient is phenylephrine hydrochloride. Each Minims unit contains approximately 0.5 ml eye drops solution of Phenylephrine hydrochloride 2.5% w/v (12.5 mg) or 10% w/v (50 mg).
The other ingredients are sodium metabisulphite (E223), disodium edetate and purified water.
This medicine does not contain a preservative as it is a sterile single use unit.
Contents of the pack Each carton contains 20 units.
Marketing Authorisation Holder: Manufacturer:
Bausch & Lomb UK Ltd. Laboratoire Chauvin - Z.I.Ripotier - 07200 Aubenas - France
106 London Road - Kingston-upon-Thames - KT2 6TN - UK Tel:+44(0)20 8781 2900 / Fax:+44(0)20 8781 2901 Marketing Authorisation Numbers:
Minims® Phenylephrine Hydrochloride 2.5% w/v: PL03468/0076 (UK); PA 555/14/2 (Ireland)
Minims® Phenylephrine Hydrochloride 10% w/v: PL03468/0077 (UK); PA 555/14/1 (Ireland)
This leaflet was last revised in: May 2016
PlUKIE03_76922_Minims-Phenylephrine_Hyd.rochIoride_2-5_10_WV_DRP_20SDU_UK_IE_030616_06.indd 2