Mirtazapine 15mg Orodispersible Tablets
Focus Pharmaceuticals Ltd
PACKAGE LEAFLET: INFORMATION FOR THE USER
Mirtazapine 15mg, 30mg and 45mg Orodispersible Tablets
Read all of this leaflet carefully before you start taking this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Mirtazapine Orodispersible Tablets are and what they are used for
2. Before you take Mirtazapine Orodispersible Tablets
3. How to take Mirtazapine Orodispersible Tablets
4. Possible side effects
5. How to store Mirtazapine Orodispersible Tablets
6. Further information
-®-
1. What Mirtazapine Orodispersible
Tablets are and what they are used for
Mirtazapine belongs to a group of medicines called antidepressants and is used to treat depression.
It may take 2 to 4 weeks before you start to feel or sleep better. It is important to take Mirtazapine every day and not to stop taking it unless your doctor tells you to. If you do, your symptoms may come back.
<S)-
2. Before you take Mirtazapine Orodispersible Tablets
Do not take Mirtazapine
If you are allergic (hypersensitive) to Mirtazapine or any of the other ingredients of Mirtazapine Orodispersible Tablets (see section 6). If you are taking or have recently taken (within the last two weeks) medicines called monoamine oxidase inhibitors (MAOIs).
Take special care with Mirtazapine if you have or have had:
• epilepsy (seizures or fits)
• kidney or liver disease (including jaundice)
• heart disease or a family history of heart disorders
• other heart conditions including heart attack, irregular heartbeat or heart failure, or are taking medicines that may affect the rhythm of your heart
• low blood pressure
• difficulty in passing water (urinating), which may be caused by an enlarged prostate
• eye disease, such as glaucoma
• diabetes
• psychiatric disorders such as schizophrenia or manic depression
In rare cases there may be changes in the white blood cells. If you get fever, sore mouth or throat and recurrent minor infections such as coughs and colds tell your doctor as you may need to stop taking your medicine. If you are an elderly person you could be more sensitive to the side effects of antidepressants. Children and adolescents under 18 years of age Mirtazapine should not normally be used for children and adolescents under 18 years because efficacy was not demonstrated. Also you should know that patients under 18 have an increased risk of side effects such as suicide attempt, suicidal thoughts and hostility (mainly aggression, oppositional behaviour and anger) when they take this type of medicine. Despite this, your doctor may prescribe Mirtazapine for patients under 18 because they have decided that this is in their best interests. If your doctor has prescribed Mirtazapine for a patient under 18 and you want to discuss this, please go back to your doctor. You should inform your doctor if any of the symptoms listed above develop or worsen when patients under 18 are taking Mirtazapine. Also, the long-term safety effects concerning growth, maturation and cognitive and behavioural
development in this age group have not yet been demonstrated. In addition, significant weight gain has been observed in this age category more often when treated with Mirtazapine compared with adults.
Thoughts of suicide and worsening of your depression
If you are depressed you can sometimes have thoughts of harming or killing yourself. These may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
if you have previously had thoughts about killing or harming yourself.
if you are a young adult. Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straight away.
You may find it helpful to tell a relative or close friend
that you are depressed, and ask them to read this leaflet. You might ask them to tell you if they think your depression is getting worse, or if they are worried about changes in your behaviour.
Taking other medicines:
Please inform your doctor or pharmacist if you are taking or have recently taken any other medicines even those not prescribed.
• Other antidepressants:
You should not take Mirtazapine Orodipsersible Tablets if you are taking other antidepressants known as Monoamine Oxidase Inhibitors (MAOIs) or in the two weeks after they have been stopped The use of other antidepressants or medicines containing the product serotonin can lead to the development of serotonin syndrome and should be used with caution.
• Drugs for anxiety or insomnia:
Mirtazapine can increase the drowsiness caused by benzodiazepines.
• Take care when taking any of the following medicines:
- drugs used in the treatment of HIV
- antibiotics such as erythromycin or rifampicin
- antifungal agents such as ketoconazole
- nefazodone, an antidepressant
- drugs for epilepsy e.g. phenytoin or carbamazapine
- cimetidine a drug used to treat indigestion or stomach ulcers
- drugs to prevent blood clotting e.g. warfarin Taking Mirtazapine with food and drink
You may get drowsy if you drink alcohol while you are taking Mirtazapine. It is therefore advisable to avoic drinking any alcohol.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking this medicine. Limited experience with Mirtazapine administration to pregnant women does not indicate an increased risk. However, caution should be exercised when used during pregnancy If you are taking Mirtazapine and you become pregnant or you plan to get pregnant, ask your doctor whether you may continue taking Mirtazapine. If you use Mirtazapine until, or shortly before birth, your baby should be supervised for possible adverse effects.
Make sure your midwife and/or doctor knows you are on Mirtazapine Orodispersible Tablets. When taken during pregnancy, similar drugs (SSRIs) may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the new born (PPHN), making the baby breathe faster and appeal bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately.
Ask your doctor whether you can breast-feed, while taking Mirtazapine.
Driving and using machines
Mirtazapine can make you feel drowsy, affect your concentration or make you less alert. When you first start taking Mirtazapine, make sure your abilities are not affected before you drive or operate machinery.
Important information about some of the ingredients of Mirtazapine
Mirtazapine Orodispersible Tablets contain a source of phenylalanine. May be harmful for people with phenylketonuria.
-:-<2H
3. How to take Mirtazapine Orodispersible Tablets
Important: only take Mirtazapine as your doctor or pharmacist tells you to. Don’t stop taking it unless your doctor tells you to.
Dosage
Adults and elderly patients:
The usual starting dose is 15mg or 30mg every day taken preferably in the evening. Your doctor may advise you to increase your dose after a few days to the amount that may be best for you (between 15 and 45mg per day).
The dose is usually the same for all ages. However, if you are an elderly person or if you have renal or liver disease, your doctor may adapt the dose.
Duration of treatment
After 2 to 4 weeks, talk to your doctor about the effect the treatment has had. If you still don’t feel well, your doctor may prescribe a higher dose. After another 2 to 4 weeks talk to your doctor again.
Method and route of administration
Take Mirtazapine at the same time each day. Your doctor will probably advise you to take Mirtazapine as a single dose before you go to bed. However, your doctor may suggest you split your dose - for example once in the morning, and once before you go to bed. The higher dose should be taken before you go to bed
Take the tablets as follows:
Take your tablet orally.
Fig A
Keep your hands dry.
Do not push the tablet out of the pocket (fig A). You will crush it.
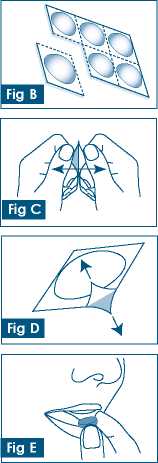
Fig B
Each strip contains six tablets.
The tablets are in pockets separated by perforations.
Tear off one tablet pocket along the dotted line (Fig B).
Fig C
arefully peel off the foil starting at the corner with the black arrow (Figs C and D)
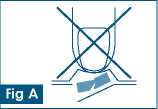
Fig E
Put the tablet on your tongue. The tablet will disintegrate and an be swallowed with or without water.
f you take more Mirtazapine than you should:
all a doctor straight away or go immediately to the nearest casualty department, taking the remaining tablets with you. The most likely signs of overdose are drowsiness and disorientation.
If you forget to take Mirtazapine:
If you are supposed to take your dose once a day: do not take a double dose to make up for forgotten ndividual doses. Just skip that dose and take your next one at the normal time.
If you are supposed to take your dose twice a day: if you have forgotten to take your morning dose, simply take it together with your evening dose. if you have forgotten to take your evening dose, do not take it with the next morning dose; just skip it and continue with your normal morning and evening doses.
if you have forgotten to take both doses, do not attempt to make up for the missed doses. Skip both doses and continue the next day with your normal morning and evening doses.
Effects when treatment with Mirtazapine is stopped:
Do not suddenly stop taking Mirtazapine even if your depression has lifted. If you stop suddenly, you may feel sick, anxious or agitated and have headaches. It s possible that some of your symptoms may come back. Once you are feeling better, talk to your doctor who will tell you how to reduce the dose gradually. This will usually be about 4 to 6 months after you start feeling better.
-&T
4. Possible side effects
Like all medicines, Mirtazapine can have side effects. Please remember that it can sometimes be hard to tell the difference between some of the milder side ffects and the symptoms of your depression.
Serious side effects
If you experience any of the following events you should tell your doctor immediately:
Very common (affects more than 1 user in 10): increase in appetite and weight gain drowsiness or sleepiness headache dry mouth Common (affects 1 to 10 users in 100): lethargy dizziness
shakiness or tremor nausea diarrhoea vomiting
rash or skin eruptions (exanthema) pain in your joints (arthralgia) or muscles (myalgia) back pain
feeling dizzy or faint when you stand up suddenly (orthostatic hypotension)
swelling (typically in ankles or feet) caused by fluid retention (oedema) tiredness vivid dreams confusion feeling anxious sleeping problems
In children under 18 years the following adverse events were observed commonly in clinical trials: significant weight gain, hives and increased blood triglycerides Uncommon (affects 1 to 10 users in 1,000): feeling elated or emotionally ‘high’ (mania)
Stop taking Mirtazapine and tell your doctor straight away.
abnormal sensation in the skin e.g. burning, stinging, tickling or tingling (paraesthesia) restless legs fainting (syncope)
sensations of numbness in the mouth (oral hypoaesthesia) low blood pressure nightmares feeling agitated
• hallucinations
• urge to move
Rare (affects 1 to 10 users in 10,000):
• yellow colouring of eyes or skin; this may suggest disturbance in liver function (jaundice)
• aggression Stop taking Mirtazapine and tell your doctor straight away.
• inflamed pancreas causing abdominal pain, swelling or tenderness (pancreatitis)
Stop taking Mirtazapine and tell your doctor straight away.
• muscle twitching or contractions (myoclonus)
Not known (cannot be estimated from the available data):
• signs of infection such as sudden unexplainable high fever, sore throat and mouth ulcers (agranulocytosis)
• increased salivation
• sleepwalking
• impaired speech Stop taking Mirtazapine and contact your doctor straight away for a blood test.
In rare cases Mirtazapine can cause disturbances in the production of blood cells (bone marrow depression). Some people become less resistant to infection because Mirtazapine can cause a temporary shortage of white blood cells (granulocytopenia). In rare cases Mirtazapine can also cause a shortage of red and white blood cells, as well as blood platelets (aplastic anaemia), a shortage of blood platelets (thrombocytopenia) or an increase in the number of white blood cells (eosinophilia).
• epileptic attack (convulsions)
Stop taking Mirtazapine and tell your doctor straight away.
• a combination of symptoms such as inexplicable fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering overactive reflexes, restlessness, mood changes and unconsciousness. In very rare cases these can be signs of serotonin syndrome.
Stop taking Mirtazapine and tell your doctor straight away.
• thoughts of harming or killing yourself Contact your doctor or go to a hospital straight away.
• abnormal sensations in the mouth (oral paraesthesia)
• swelling in the mouth (mouth oedema)
• severe rashes that may involve blistering and peeling of the skin (Stevens-Johnson syndrome)
• blistering or scaling decay of the skin
• blistering rash
• round red patches on the skin
• hyponatraemia
• inappropriate anti-diuretic hormone secretion
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
<j>
5. How to store Mirtazapine Orodispersible Tablets
Keep out of the reach and sight of children.
Store in the original package in order to protect from moisture.
Do not use after the expiry date stated on the carton or foil.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
<D-
6. Further information
What Mirtazapine Orodispersible Tablets contain
• The active substance is Mirtazapine. Each tablet contains either 15mg, 30mg and 45mg of the active ingredient Mirtazapine.
• The other ingredients are mannitol, microcrystalline cellulose, crospovidone, hydroxylpropyl cellulose magnesium carbonate heavy, silica colloidal anhydrous, L-methionine, magnesium stearate guar gum, aspartame (E951), orange flavour.
Each Mirtazapine Orodispersible Tablet is a white round, biconvex, uncoated tablet with the following markings:
15mg orodispersible tablets - M1 30mg orodispersible tablets - M2 45mg orodispersible tablets - M4 The tablets are supplied in blister packs of 30 Tablets.
Marketing Authorisation holder and manufacturer Marketing Authorisation Holder:
Focus Pharmaceuticals Limited, Capital House, 1st Floor, 85 King William Street, London EC4N 7BL, United Kingdom.
Manufactured by:
Actavis Limited, BLB016, Bulebel Industrial Estate, Zejtun ZTN 3000 Malta.
This leaflet was last revised in 06/2015.
|
FOCUS _ |
tel: 01283 495280 fax: 01283 495290 |
CLEARLY MARK ANY AMENDMENTS AND RESUBMIT Approved _/\_ Amend Z' N. for ^ 7 & Print Resubmit V___7 |
FOCUS VERSION 13 01/06/2015 | |
|
Focus Contact: |
Signature: (Proof Reader) Date: |
Signature: (Regulatory) Date: | ||
|
Product Description/Component: Mirtazapine Orodispersible Tablets - PIL |
Signature: (Marketing) Date: |
Signature: (Braille) Date: | ||
|
Market: UK / English |
File Name: Mirtazapine_TAB_PIL |
Part No/Component Code: | ||
|
Supplier / Manufacturer: XXXXX |
Printer / Repro: XXXXX |
Software Package: Adobe Illustrator CS3 | ||
|
Substrate: Paper |
Cutter Ref: |
PL No.: PL 20046/0062 - 0064 | ||
|
Barcode: |
Dimensions: 170 x 450 mm (Portrait) |
Minimum Point Size: 9pt Headers/8pt Body Text | ||
|
Keys: Dieline Print Free (^) |
Pantone/ Process Cols Used: P 302 | |||
|
Reason for New Version: v7 Error in sizing corrected 400mm > 450mm length. v8 approval date removed and Actavis Iceland (man) re-instated. v9 Actavis Iceland Man. address removed & approval date added. v10 Text addition to section 4 & addition of new PV statement. v11 Text additions to sections 2 & 4, and amendment of PV statement. v12 removal of paragraph prior to PV statement. v13 MAH address changed | ||||
|
Caution! We cannot accept responsibility for any errors in this proof after approval. The artwork received has been significantly adjusted, revised or reset by us from disk or hard copy. Whilst we take extreme care at all times to ensure accuracy, the final responsibility must be taken by our customer. If you sign this proof you are signifying full approval of design and text. | ||||