Mitomycin 40 Mg Powder And Solvent For Intravesical Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Mitomycin 40 mg, Powder and solvent for intravesical solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial of Mitomycin contains 40 mg mitomycin.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for intravesical solution
Powder: Grey to grey blue powder or cake. Solvent: Clear and colourless solution
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Mitomycin is indicated as intravesical administration for relapse prevention in adult patients with superficial urinary bladder carcinoma after transurethral resection.
4.2 Posology and method of administration
Mitomycin must be administered by physicians experienced in this therapy, only if strictly indicated.
Mitomycin is intended for intravesical use following reconstitution.
Posology
There are many intravesical mitomycin regimens, varying in dose of mitomycin used, the frequency of instillation and the duration of therapy.
Unless otherwise specified, the dosage of mitomycin is 40 mg mitomycin instilled into the bladder once weekly. Regimens with instillations every 2 weeks, every month or 3 monthly can also be used.
The specialist should decide on the optimum regime, frequency and duration of therapy on an individual patient basis.
The urine pH should be higher than pH 6.
Special populations
The dose must be reduced in patients who have undergone extensive previous cytostatic therapy, in case of myelosuppression or in elderly patients.
Insufficient data from clinical studies are available concerning the use of mitomycin in patients >65 years of age.
The product should not be used in patients with renal impairment (see section 4.3)
The product is not recommended in patients with hepatic impairment due to lack of efficacy and safety data in this group of patients.
Paediatric population
The safety and efficacy of mitomycin in children have not been established. No data are available.
Method of administration
Mitomycin is intended for intravesical instillation after being dissolved.
For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Breastfeeding
Bladder wall perforation
Cystitis
4.4 Special warnings and precautions for use
Due to the toxic effects on the bone marrow of mitomycin, other myelotoxic therapy modalities (in particular other cytostatics, radiation) must be administered with particular caution in order to minimise the risk of additive myelosuppression.
Long-term therapy may result in cumulative bone marrow toxicity. Bone marrow suppression may only manifest itself after a delay, being expressed most strongly after 4 -6 weeks, accumulating after prolonged use and therefore often requiring an individual dose adjustment.
Elderly patients often have reduced physiological function, bone marrow depression, which may be protracted, so administer mitomycin with special caution in this population while closely monitoring patient's condition.
Mitomycin is a mutagenic and potentially carcinogenic substance in humans. Contact with the skin and mucous membranes is to be avoided.
In the case of pulmonary symptoms, which cannot be attributed to the underlying disease, therapy should be stopped immediately. Pulmonary toxicity can be well treated with steroids.
Therapy should be stopped immediately also if there are symptoms of haemolysis or indications of renal dysfunction (nephrotoxicity). The occurrence of a haemolytic-uraemic syndrome (HUS: irreversible renal failure, microangiopathic haemolytic anaemia [MAHA syndrome] and thrombocytopenia) is commonly fatal.
At doses of > 30 mg of mitomycin/m2 of body surface microangiopathic-haemolytic anaemia has been observed. Close monitoring of renal function is recommended.
New findings suggest a therapeutic trial may be appropriate for the removal of immune complexes that seem to play a significant role in the onset of symptoms by means of staphylococcal protein A.
Occurrence of acute leukaemia (in some cases following preleukaemic phase) and myelodysplastic syndrome has been reported in the patients treated concomitantly with other antineoplastic agents.
4.5 Interaction with other medicinal products and other forms of interaction Possible interaction under systemic therapy
Myelotoxic interactions with other bone marrow-toxic treatment modalities (especially other cytotoxic medicinal products, radiation) are possible.
Combination with vinca alkaloids or bleomycin may reinforce pulmonary toxicity.
An increased risk of haemolytic-uremic syndrome has been reported in patients receiving a concomitant administration of mitomycin and 5-fluorouracil or tamoxifen.
In animal experiments, pyridoxine hydrochloride (vitamin B6) resulted in the loss of effect of mitomycin.
No injections with live vaccines should be carried out in connection with mitomycin treatment, as this may result in an increased risk of infection by the live vaccine.
The cardiotoxicity of doxorubicin may be reinforced by mitomycin.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no data from the use of mitomycin in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). Mitomycin has a mutagenic, teratogenic and carcinogenic effect and therefore may impair the development of an embryo.
Women must not become pregnant during treatment with mitomycin. In the event of pregnancy during treatment, genetic counselling must be provided.
Breastfeeding
It is suggested that mitomycin is excreted in breast milk. Due to its proven mutagenic, teratogenic and carcinogenic effects, breastfeeding must be discontinued during treatment with mitomycin (see section 4.3).
Fertility
Sexually mature patients should take contraceptive measures or practise sexual abstinence during chemotherapy and for 6 months afterwards.
Mitomycin is genotoxic. Men who are being treated with mitomycin are therefore advised not to father a child during treatment and up to 6 months thereafter and to seek advice on the preservation of sperm before the start of therapy due to the possibility of irreversible infertility caused by the therapy with mitomycin.
4.7 Effects on ability to drive and use machines
Even when used in accordance with instructions these medicinal product may cause nausea and vomiting and thereby reduce reaction times to such an extent that the ability to drive a motor vehicle or operate machinery is impaired. This applies even more in connection with alcohol.
4.8 Undesirable effects
Undesirable effects are listed below by system organ class and frequency. Frequencies below are defined as:
Very common (> 1/10), common (> 1/100 to < 1/10), uncommon (> 1/1,000 to < 1/100), rare (> 1/10,000 to < 1/1,000), very rare (< 1/10,000) or not known (cannot be estimated from the available data).
Possible side-effects under intravesical therapy
Adverse reactions may result either from the solution for intravesical instillation or after deep resection.
The most common undesirable effects of intravesically administered mitomycin are allergic skin reactions in the form of local exanthema (e.g. contact dermatitis, also in the form of palmar and plantar erythema), and cystitis.
|
Skin and subcutaneous tissue disorders |
Common Allergic skin rash, contact dermatitis, palmar-plantar erythema, pruritus Rare Generalised exanthema |
|
Renal and urinary disorders |
Common Cystitis (possibly haemorrhagic), dysuria, nocturia, pollakiuria, haematuria, local irritation of the bladder wall Very rare or not known Necrotising cystitis, allergic (eosinophilic) cystitis, stenosis of the efferent urinary tract, reduced bladder capacity, bladder wall calcification, bladder wall fibrosis, bladder perforation |
If cystitis does occur, symptomatic treatment with local anti-inflammatories and analgesics should be given. In most cases the mitomycin therapy can be continued, if necessary at a reduced dose. Isolated cases of allergic (eosinophilic) cystitis have been reported which necessitated discontinuation of therapy.
After intravesical administration, only minor amounts of mitomycin reach the systemic circulation. Nevertheless, in very rare cases the following systemic undesired effects have been reported:
Possible systemic undesirable effects occurring very rare following intravesical administration
|
Blood and lymphatic system disorders |
Leukocytopenia, thrombocytopenia |
|
Respiratory, thoracic and mediastinal disorders |
Interstitial lung disease |
|
Gastrointestinal disorders |
Nausea, vomiting, diarrhoea |
|
Hepato-biliary disorders |
Transaminases increased |
|
Skin and subcutaneous tissue disorders |
Alopecia |
|
Renal and urinary disorders |
Renal dysfunction |
|
General disorders and administration site conditions |
Fever |
Possible side-effects under systemic therapy
The most common side effects of mitomycin administered systemically are gastrointestinal symptoms like nausea and vomiting and bone marrow suppression with leukopenia and mostly dominant thrombocytopenia. This bone marrow suppression occurs in up to 65 % of patients.
In up to 10 % of patients serious organ toxicity in the form of interstitial pneumonia or nephrotoxicity must be expected.
Mitomycin is potentially hepatotoxic.
|
Blood and lymphatic system disorders |
Very common Bone marrow suppression, leucopenia, thrombocytopenia Rare Life-threatening infection, sepsis, haemolytic anaemia |
|
Immune system disorders |
Very rare Severe allergic reaction |
|
Cardiac disorders |
Rare Heart failure after previous therapy with anthracyclines |
|
Respiratory, thoracic and mediastinal disorders |
Common Interstitial pneumonia, dyspnoea, cough, shortness of breath Rare |
|
Pulmonary hypertension, pulmonary veno-occlusive disease (PVOD) | |
|
Gastrointestinal disorders |
Very common Nausea, vomiting Uncommon Mucositis, stomatitis, diarrhoea, anorexia |
|
Hepato-biliary disorders |
Rare Liver dysfunction, increased transaminases, jaundice, veno-occlusive disease (VOD) of the liver |
|
Skin and subcutaneous tissue disorders |
Common Exanthema, allergic skin rash, contact dermatitis, palmar-plantar erythema Uncommon Alopecia Rare Generalised exanthema |
|
Renal and urinary disorders |
Common Renal dysfunction, increase in serum creatinine, glomerulopathy, nephrotoxicity Rare Haemolytic uraemic syndrome (HUS) (commonly fatal), microangiopathic-haemolytic anaemia (MAHA syndrome) |
|
General disorders and administration site conditions |
Common Following Extravasation: Cellulitis, tissue necrosis Uncommon Fever |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
In case of overdose severe myelotoxicity or even myelophthisis must be expected, with the full-blown clinical effect only appearing after approximately 2 weeks.
The period until which the number of leucocytes falls to the lowest value may be 4 weeks. Prolonged close haematological monitoring therefore also has to be carried out if an overdose is suspected.
However, up till now, no cases of overdose of intravesical administration of mitomycin have been reported.
As no effective antidote is available, the utmost caution should be exercised at each administration.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, cytotoxic antibiotics and related substances, other cytotoxic antibiotics, ATC code: L01DC03
The antibiotic mitomycin is a cytostatic medicinal product from the group of alkylating agents.
Mitomycin is an antibiotic with an antineoplastic effect which is isolated from Streptomyces caespitosus. It is present in an inactive form. Activation to a trifunctional alkylating agent takes place rapidly, either at physiological pH in the presence of NADPH in serum or intracellularly in virtually all cells of the body with the exception of the cerebrum, as the blood-brain barrier is not overcome by mitomycin. The three alkylating radicals all stem from a quinone, an aziridine and a urethane group. The mechanism of action is based predominantly on DNA (to a lesser extent RNA) alkylation, with the corresponding inhibition of DNA synthesis. The degree of DNA damage correlates with the clinical effect and is lower in resistant cells than in sensitive cells. As with other alkylating agents, proliferating cells are damaged to a greater extent than those in the resting phase (G0) of the cell cycle. Additionally, free peroxide radicals are released, particularly in the case of higher doses, which result in DNA breaks. The release of peroxide radicals is associated with the organ-specific pattern of side effects.
5.2 Pharmacokinetic properties
Following intravenous administration of 10-20 mg/m2 mitomycin, peak plasma levels of 0.4- 3.2 pg/ml have been measured. The biological half life is short, between 40 and 50 minutes. The serum level falls biexponentially, steeply within the first 45 minutes and more slowly thereafter.
After approximately 3 hours the serum levels are usually below the detection limit. The main location for metabolism and elimination is the liver. Accordingly, high
concentrations of mitomycin have been found in the gall bladder. Renal excretion plays only a minor role with respect to the elimination.
During intravesical therapy mitomycin is only absorbed in insignificant doses. Nevertheless, a systemic effect cannot be excluded completely.
5.3 Preclinical safety data
In animal studies mitomycin has a toxic effect on all proliferating tissues, in particular on the cells of the bone marrow and the gastrointestinal mucosa, and spermatogenesis is inhibited.
Mitomycin has mutagenic, carcinogenic and teratogenic properties, which can be demonstrated in appropriate experimental models.
If injected outside a vein, or in the event of extravasation into surrounding tissue, mitomycin causes severe necrosis.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Powder for solution for intravesical use: Urea
Solvent for intravesical solution: Sodium chloride and water for injections.
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life
Mitomycin vials with 40 mg mitomycin and instillations set 9 months
From a chemical and physical point of view the reconstituted product should be used within 24 hours.
From a microbiological point of view, unless the method of opening/reconstitution/dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4 Special precautions for storage
Do not store above 25 °C. Store the vial in the outer carton in order to protect the contents from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Mitomycin is contained within clear glass vials (type I) with fluoropolymer coated bromobutyl rubber stopper and a flip off aluminium seal.
Packs of 1 vial (50 ml), 1 PVC bag of 40 ml with 0.9 % sodium chloride solution, catheters
Packs of 4 vials (50 ml), 4 PVC bags of 40 ml with 0.9 % sodium chloride solution, catheters
Packs of 5 vials (50 ml), 5 PVC bags of 40 ml with 0.9 % sodium chloride solution, catheters
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Dissolve the content of one vial of Mitomycin (equivalent to 40 mg mitomycin) in 40 ml sterile 0.9 % sodium chloride solution. The contents of the vial must dissolve to form a blue-purple clear solution within 2 minutes.
Only clear solutions may be used.
The content of the vials is intended for single use/single entry only. Unused solution must be discarded.
Protect the reconstituted solution from light.
Mitomycin must not be used in mixed injections. Other solutions for injection or infusion must be administered separately.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for use for the solvent for intravesical solution (instillation set)
Fig. 1 - 7:
(1)
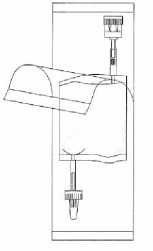
Tear open the protective cover, but do not remove completely! This will keep the tip of the instillation system protected from contamination until the last minute.
(2)
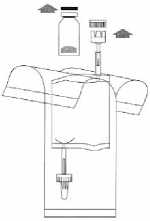
Remove the caps from the vial and the instillation system. Place the disposal bag ready to hand.
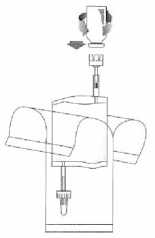
Holding the vial vertically, push it firmly onto the adapter of the instillation system and turn once or twice.
(4)
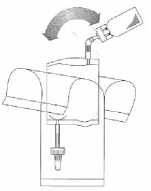
Holding the tube (not the vial) firmly in the vertical position, break the upper valve by bending it backwards and forwards.
(5)
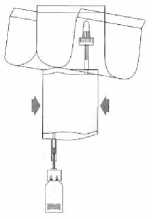
Pump the liquid into the vial, but do not fill the vial completely.
(6)
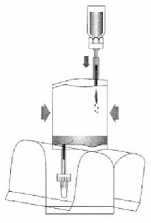
Invert the entire system. Hold the vial up and pump in air. Draw the dissolved mitomycin into the instillation system. Do not remove the vial.
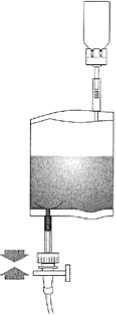
Hold the instillation system vertically. Now remove the protective cover completely. Connect the catheter to the system. Now break the sealing mechanism in the tube section by bending backwards and forwards and instil the solution. After completing the instillation, release the catheter by squeezing air through. Keep the solvent bag squeezed together and transfer with the catheter to the disposal bag.
7 MARKETING AUTHORISATION HOLDER
PHARMALEX GMBH
JOSEPH-MAYER-STRASSE 13-15
MANNHEIM
D-68167
GERMANY
8 MARKETING AUTHORISATION NUMBER(S)
PL 27904/0007
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16/12/2015
10 DATE OF REVISION OF THE TEXT
16/12/2015