Mometasone Furoate 50 Micrograms/Actuation Nasal Spray Suspension
Out of date information, search anotherPackage leaflet: Information for the user Mometasone Furoate 50 micrograms/actuation nasal spray.
suspension
(mometasone furoate as the monohydrate)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have further questions, please ask your doctor or pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What Mometasone furoate 50 micrograms/actuation nasal spray, suspension is and what it is used for
2. What you need to know before you use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
3. How to use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
4. Possible side effects
5. How to store Mometasone furoate 50 micrograms/actuation nasal spray, suspension
6. Contents of the pack and other information
1. What Mometasone furoate 50 micrograms/actuation nasal spray, suspension is and what it is used for
Mometasone furoate 50 micrograms/actuation nasal spray, suspension contains the active ingredient mometasone furoate (as the monohydrate). Mometasone furoate is a corticosteroid (steroid for short) which has an anti-inflammatory action, reducing swelling and irritation which causes sneezing, itching and a blocked or runny nose.
Each spray provides 50 micrograms of the active ingredient mometasone furoate (as the monohydrate).
This medicine is used to prevent and treat seasonal allergic rhinitis (e.g. hay fever) and perennial rhinitis (e.g. year round rhinitis often due to house dust mites or animal allergies) in adults and children 6 years of age and older.
Mometasone furoate 50 micrograms/actuation nasal spray, suspension is also used to treat nasal polyps in adults 18 years of age and over. Nasal polyps are small growths on the lining of the nose and usually affect both nostrils. The main symptom is a blocked feeling in the nose which may affect breathing through the nose. Watering from the nose, a feeling of something running down the back of the throat and loss of taste and smell may also occur. Mometasone furoate reduces the inflammation in the nose, causing the polyps to gradually shrink.
2. What you need to know before you use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
Do not use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
- if you are allergic (hypersensitive) to mometasone furoate or to any of the other ingredients of this medicine (listed in section 6).
- if you have an infection in your nose. You should wait until the infection is resolved before you start using the nasal spray.
- If you have recently had an operation on your nose or have injured your nose. You should wait until it has healed before you start using the nasal spray.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Mometasone furoate 50 micrograms/actu-ation nasal spray, suspension if you:
- are pregnant, likely to become pregnant or if you are breast-feeding a baby.
- have or have ever had tuberculosis.
- have herpes simplex (virus) infection of the eye.
- have any other type of infection.
- are taking other corticosteroid medicines, either by mouth or by injection.
- have cystic fibroisis.
Whilst using Mometasone furoate 50 micrograms/actuation nasal spray, suspension, avoid coming into contact with anyone who has measles or chickenpox. You should tell your doctor if you do come into contact with anyone who is suffering from these infections.
Children and adolescents
It is recommended that the height of children receiving long-term treatment with nasal corticosteroids is regularly monitored and if any changes are noted, you should notify your doctor.
Other medicines and Mometasone furoate 50 micrograms/actuation nasal spray, suspension
Please tell your doctor if you are taking, have recently taken, or might take any other medicines. This includes medicines bought without a prescription.
Tell your doctor or pharmacist if you are currently taking any of the following medicines before using Mometasone furoate 50 micrograms/actuation nasal spray, suspension:
- any other corticosteroid medicines for allergy treatment, either by mouth or injection
Page 1
A few people may find that once they discontinue oral or injected cortico-steroids they suffer from some undesirable effects, such as joint or muscular pain, weakness and depression. If these occur you should inform your doctor who will advise about continuing use of your nasal spray. You may also seem to develop other allergies, such as itchy, watering eyes or patches of red and itchy skin. If you develop any of these effects and you are worried, you should see your doctor.
Mometasone furoate 50 micrograms/actuation nasal spray, suspension with food, drink and alcohol
Food and drink have no known influence on this medication.
Pregnancy and breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Mometasone furoate 50 micrograms/actuation nasal spray, suspension contains mometasone furoate which is not known to affect ability to drive or operate machinery. If you experience dizziness or other similar side effects you should not drive or use machines whilst using mometasone furoate.
Mometasone furoate 50 micrograms/actuation nasal spray, suspension contains benzalkonium chloride
Mometasone furoate 50 micrograms/actuation nasal spray, suspension contains benzalkonium chloride which may cause skin reactions or nasal irritation. If used for a prolonged period, benzalkonium chloride may cause swelling of the nasal mucosa leading to nasal congestion. If nasal congestion persists, see your doctor.
3. How to use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
ONLY USE IN YOUR NOSE.
For Seasonal or Perennial Allergic Rhinitis
Adults, the elderly and children 12 years of age and older
The recommended dose is TWO sprays into each nostril ONCE a day, taken in the morning.
Your doctor may increase this to a maximum of TWO sprays TWICE a day.
If your symptoms become better, you doctor may lower the dose to ONE spray into each nostril ONCE a day. However, if you do not start to feel any better, you should see your doctor and he may tell you to increase the dose to the maximum daily dose of four sprays into each nostril once a day. Once your symptoms are controlled, your doctor may advise you to reduce the dose to two sprays into each nostril once daily.
Use in children 6 -11 years of age
ONE spray into each nostril ONCE a day, taken in the morning.
Long term use of nasal steroids at high does may cause slowing of growth in children. Your doctor may check your child’s height at intervals during treatment and reduce the dose if any effects are seen.
If you suffer badly from hayfever, your doctor may tell you to start using Mometasone furoate 50 micrograms/actuation nasal spray, suspension two to four weeks before the start of the pollen season, as this will help to prevent your hayfever symptoms from occurring.
At the end of the pollen season your hayfever symptoms should get better and treatment may then not be needed.
If you also have itchy, watery eyes, you should tell your doctor. He or she may give you an extra medicine to treat your eyes.
For nasal polyps Adults aged 18 and over
TWO sprays into each nostril ONCE daily.
If symptoms are not controlled after 5 to 6 weeks, the dose may be increased to two sprays in each nostril twice daily. Once symptoms are under control, your doctor should ask you to reduce your dose. If no improvement in symptoms is seen after 5 to 6 weeks of twice daily administration you should contact your doctor to discuss other treatments to replace Mometasone furoate 50 micrograms/actuation nasal spray, suspension.
Preparing your nasal spray for use
If you are using the nasal spray for the first time, you should prime the pump by pumping the spray 10 times until a fine mist is produced. To do this:
1. Gently shake the bottle
2. Put your forefinger and middle finger on the collar either side of the nozzle and your thumb underneath the bottle.
3. Keep the thumb still and press down with your fingers to pump the spray, holding the nozzle away from you.
If you have not used the nasal spray for 14 days or longer, you should re-prime the pump by pumping the spray 2 times until a fine mist is produced.
If there is no spray and you think the nozzle may be blocked, clean it. (Refer to cleaning instructions below). DO NOT try to unblock the spray hole with a pin or other sharp object as this will destroy the spray mechanism.
CO
co
co
O
C\l
Page 2
UK
NASAL
(DCP+RUP+Additional BRS)
o
o
h-
° -■
i ^


o
09
o
£ <= -3
■o ^
O
o
o
o
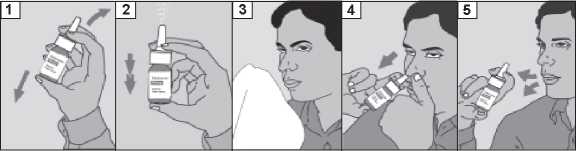
How to use the Spray
4. Possible side effects
United Kingdom Bulgaria
Slovak Republic
Estonia
Greece
Iceland
Netherlands
Romania
Malta
Cyprus
Ireland
Luxembourg
Czech Republic Poland
1. Shake the bottle.
2. Remove the dust cap.
3. Blow your nose gently.
4. Hold the bottle as shown. Place your finger on your nose to close one nostril, and put the nozzle in your other nostril. Tilt your head forward and keep the bottle upright. Start to breathe in slowly through your nose and at the same time press down firmly on the collar of the bottle with your finger, which releases a spray of mometasone furoate.
5. Breathe out through your mouth. Repeat step 4 if more than one spray is required into the same nostril.
Remove the nozzle from the nostril and breathe out through your mouth.
Repeat steps 4 to 5 for the other nostril.
After using the spray, wipe the nozzle carefully with a clean tissue and replace the dust cap. Cleaning your Mometasone furoate 50 micrograms/actuation nasal spray, suspension bottle
1. Clean your nasal spray at least once a week as it can become blocked, stopping it from working properly.
2. Remove the dust cap.
3. Gently pull upwards on the white collar to remove the nozzle.
4. Soak the nozzle and dust cap in warm water for a few minutes, and then rinse under a running tap.
5. Allow to dry in a warm place before putting back together. Do not dry near a very hot place.
6. Re-fit the nozzle and ‘prime’ the bottle if necessary by pumping the spray a few times to produce a fine mist.
7. Do not try to unblock the nasal applicator by inserting a pin or other sharp object as this will damage the applicator and cause you not to get the right dose of medicine.
If you use more Mometasone furoate 50 micrograms/actuation nasal spray, suspension than you should
If you accidentally use more than your prescribed dose of Mometasone furoate 50 micrograms/actuation nasal spray, suspension tell your doctor as soon as possible.
If you forget to use Mometasone furoate 50 micrograms/actuation nasal spray, suspension
If you forget a dose of Mometasone furoate 50 micrograms/actuation nasal spray, suspension, use it as soon as you remember, then carry on as before. Do not take an extra dose to make up for the one that you missed.
If you stop using Mometasone furoate 50 micrograms/actuation nasal spray, suspension
In some patients Mometasone furoate 50 micrograms/actuation nasal spray, suspension should begin to relieve symptoms 12 hours after the first dose; however full benefit of treatment may not be seen for up to two days. It is very important that you use your nasal spray regularly. Do not stop your treatment even if you feel better unless told to do so by your doctor.If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
Like all medicines, this medicine can cause side effects, although not everybody gets them. Rarely, immediate hypersensitivity (allergic) reactions including Breathing difficulty caused by narrowing of airways (bronchospasm) and difficulty in breathing (dyspnoea) may occur after use of this product. Very rarely, whole body severe allergic reaction (anaphylaxis) and giant hives (angioedema) have been reported. Very rarely, these reactions may be severe. If you have any wheezing or have trouble breathing, you should stop using this medicine and get immediate medical help.
Additional side effects seen with this medicine may include:
Common may affect up to 1 in 10 people
- headaches
- sneezing
- nose bleeds
- irritation and/or dryness of the nose and throat
Uncommon may affect up to 1 in 100 people
- hypersensitivity (allergic reaction)
- difficulty breathing
- alterations in taste or smell
- rash
Very rare may affect up to 1 in 10,000 people
- increased pressure in the eye (glaucoma)
- visual disturbances
- damage to the partition in the nose which separates the nostrils.
In rare cases, treatment with corticosteroid nasal sprays like Mometasone furoate has led to an increase in pressure in the eye (glaucoma) and/or cataracts, causing visual disturbances and damage to the partition in the nose which separates the nostrils. Contact your doctor if you notice any of these side effects. Page 3
Additional side effects in children and adolescents
When used at high doses for long periods of time, corticosteroid nasal sprays may cause certain side effects, such as growth reduction in children.
Prolonged or excessive use
Prolonged or excessive use of steroids may rarely affect some of your hormones and affect growth and development in children.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: http://www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Mometasone furoate 50 micrograms/actuation nasal spray, suspension
Keep out of sight and reach of children.
Do not store this medicine above 25°C. Do not freeze this medicine.
Store in the original container.
Do not use Mometasone furoate 50 micrograms/actuation nasal spray, suspension after the expiry date which is printed on the bottle and carton after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment. Use within 2 months of first use
6. Contents of the pack and other information
What Mometasone furoate 50 micrograms/actuation nasal spray, suspension contains
- The active substance is mometasone furoate.
- The other ingredients are glycerol, microcrystalline cellulose , carmellose sodium, citric acid monohydrate, polysorbate 80, benzalkonium chloride, sodium citrate dihydrate, water for injection.
What Mometasone furoate 50 micrograms/actuation nasal spray, suspension looks like and contents of the pack
One white opaque plastic vial, fitted with a white-metered atomising pump, white nasal adaptor and a translucent dust cap for nozzle.
Each bottle of Mometasone furoate 50 micrograms/actuation nasal spray, suspension provides 140 sprays. Each actuation delivers dose of 50 micrograms of mometasone furoate (as the monohydrate).
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Cipla (EU) Limited, Hillbrow House, Hillbrow Road,
Esher, Surrey, KT10 9NW, United Kingdom
Manufacturer: Pharmadox Healthcare Ltd.,KW20A Kordin Industrial Park,
Paola, PLA 3000, Malta
S&D Pharma CZ, spol. s r.o, Pisnicka 22, 142 00 Praha 4, Ceska republika
Cipla (EU) Limited, 4th Floor, 1 Kingdom Street, London, W2 6BY, United Kingdom
Cipla Europe NV, Uitbreidingstraat 80, 2600 Antwerp, Belgium
This medicinal product is authorised in the Member States of the EEA under the following names:
Mometasone furoate 50 micrograms/actuation Nasal Spray, Suspension MoMera30HOB ^ypoar Cnnaa 50 MHKporpaMa / BnptcKBaHe cnpeft 3a hoc, cycneronfl
Mometasone furoate Cipla 50 mikrogramov nosova suspenzna aerodisperzia
Mometasone Cipla
Mometasone furoate Cipla 50 piKpoypappapia / svspyonovqan, Pwiko SKvsqxapa, svairopppa
Mometasone furoate Cipla 50 mikrogromm/uSaskammt nefudi, dreifa Mometasonfuroaat Cipla 50 microgram/ verstuiving neusspray, suspensie
Mometazona furoat Cipla 50 micrograme/doza, spray nazal, suspensie Mometasone furoate 50 micrograms/actuation Nasal Spray, Suspension Mometasone furoate Cipla 50 piKpoypappapia / svspyonovqap, Pwiko SKve^ropa, svairopppa
Mometasone furoate 50 micrograms/actuation nasal spray, suspension Mometasone furoate Cipla 50 microgrammes/ pulverisation, suspension pour pulverisation nasale
Mometason furoat Cipla 50 mikrogramu/davku Nasehaler
Portugal Mometasona Generis
This leaflet was last revised in 05/2015.
Cipla
CO
CO
CO
•'d
o
c\j
Page 4