Mydriacyl 0.5% W/V Eye Drops Solution
44285-7
II III
Package Leaflet - Information for the User
MYDRIACYL® 0.5% w/v eye drops, solution
Tropicamide
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others.
It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
1
WHAT MYDRIACYL1 0.5% IS AND WHAT IT IS USED FOR |
MYDRIACYL 0.5% belongs to a group of MYDRIACYL 0.5 % may also be used before or
medicines known as anticholinergics. after you have surgery on your eye.
It is used to enlarge the pupil of your eye (mydriasis) to allow for easier examination of the inside of the eyeball and to relax the muscles in your eye (cycloplegia) when your vision is being tested.
2
Do not use MYDRIACYL 0.5%...
• If you have or think that you may get glaucoma.
• If you are allergic to tropicamide or any of the other ingredients listed in section 6.
Ask your doctor for advice.
Take special care...
• Consult your doctor for advice; use of this product may cause:
- Increase in the pressure in your eye. You should have your eye pressure checked before initiation of therapy, especially in elderly.
- Changes in behaviour (especially in infants and children).
• If your eyes are red.
• If you are not able to use medicines called belladonna alkoloids, (you may be more likely to experience side effects).
If any of these apply, you may still be able to
use MYDRIACYL 0.5%, but discuss it with
your doctor first.
Use in children
• Do not use in concentrations greater than 0.5% in small infants because of the risk of serious adverse reactions.
• Take special care if MYDRIACYL 0.5% is being administered to infants, small or premature children, children with: Down syndrome, spastic paralysis, brain damage. Consult your doctor for advice as serious adverse reactions may occur with the use of this product.
Pregnancy and breast-feeding
If you are pregnant or might get pregnant or are breast feeding a baby, talk to your doctor before you use MYDRIACYL 0.5%.
Driving and using machines
You may experience temporary drowsiness, blurred vision and sensitivity to light when using this medicine. Do not drive or use any machines unless your vision is clear. This may take up to 6 hours after you have used MYDRIACYL 0.5%.
Using other medicines
Tell your doctor or pharmacist if you are
taking or have recently taken any other medicines, including medicines obtained without a prescription. This is particularly important if you are taking medicines for the treatment of allergies, depression, anxiety, heart disease, asthma or other breathing problems, motion sickness, Parkinson's disease, bladder or gastrointestinal problems.
Important information if you wear Contact Lenses
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in MYDRIACYL 0.5% (benzalkonium chloride) that can discolour soft contact lenses.
The usual dose
The usual dose to enlarge the pupil before examination of the inside of your eye is 1 or 2 drops in your eye(s) 15 to 20 minutes before examination.
Remove the loose collar from the cap when the bottle is first opened.
Always use MYDRIACYL 0.5% exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
HOW TO USE MYDRIACYL® 0.5% (continued)

How to use
• Wash your hands before you start.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
• Pull down your lower eyelid with a finger, until there is a ‘pocket’ between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not get MYDRIACYL 0.5% in your child’s mouth; wash your hands and your child’s hands immediately after administration.
• MYDRIACYL 0.5% may be harmful, especially in children, if too much is used or if it is accidentally swallowed. If this happens, the body surface of infants and children should be kept moist. Contact your doctor or hospital emergency department immediately.
• If a drop misses your eye, try again.
• If you use more MYDRIACYL 0.5% than you should rinse it all out with lukewarm water see your doctor as soon as possible.
Do not touch your eye or eyelid,
surrounding areas or other surfaces with the dropper. It could infect the drops.
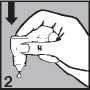
Gently press on the base of the bottle to release one drop at a time (picture 2).
Do not squeeze the bottle, only a gentle press on the bottom is needed.
Press the corner of your eye nearest to your nose with a finger for 2 minutes immediately after use. This will reduce the amount of the medicine getting to the rest of your body. Keep the eyelid closed for 2 minutes.
If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
Symptoms of overdose may include: flushing and dryness of the skin (a rash may be present in children), blurred vision, rapid and irregular pulse, fever, abdominal swelling in infants, convulsions, hallucinations, or loss of coordination.
In case of severe overdose or accidental ingestion, immediately seek advice from your doctor, pharmacist as serious reactions may occur (especially in children).
• If you are using other eye drop or eye ointment medicines, leave at least 5 minutes between each medicine. Eye ointments should be administered last.
If you have any further questions on the use
of MYDRIACYL 0.5%, ask your doctor or pharmacist.
~4| POSSIBLE SIDE EFFECTS
Like all medicines, MYDRIACYL 0.5% can cause side effects, although not everybody gets them.
• You may experience some or all of the following effects in your eye(s):
Temporary blurred vision, sensitivity to light, pain, irritation, redness, increase in pupil size, increased pressure in the eye (which may cause headache).
• You may also experience effects in other areas of your body including:
Dizziness, headache, fainting, decreased blood pressure, nausea and rash. Psychotic reactions and behavioural disturbances have been reported with this class of drug, especially in children, flushing of the skin, dryness of mucous membranes, dryness of skin, irregular heartbeat, decrease in sweating and dryness of the mouth, constipation, inability to (completely) empty the bladder.
• In children, other effects that may occur include:
Skin rash, changes in behaviour, enlarged or abnormal abdomen (in infants). More extreme reactions such as confusion, excitement, hallucinations, increasing drowsiness, rapid breathing and changes in the pulse rate require urgent medical advice and attention.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5
HOW TO STORE MYDRIACYL® 0.5%
• Stop using the bottle 4 weeks after first opening, to prevent infections.
• Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• Do not pass this medicine on to others. It may harm them, even if their symptoms are the same as yours.
6
FURTHER INFORMATION
What MYDRIACYL 0.5% contains
• The active substance is tropicamide 5 mg/ml
• The other ingredients are sodium chloride, disodium edetate, benzalkonium chloride, hydrochloric acid and/or sodium hydroxide (to adjust pH) and purified water.
What MYDRIACYL 0.5 % looks like and contents of the pack MYDRIACYL 0.5% is a clear, colourless solution supplied in a pack containing a 5 ml plastic bottle with a screw cap.
Marketing authorisation holder:
Alcon Laboratories (UK) Ltd.
Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, United Kingdom.
Manufacturer:
SA Alcon-Couvreur NV
Rijksweg 14, B-2870 Puurs, Belgium.
This leaflet was last revised in December 2014.
© 2006, 2010, 2012, 2013, 2014 Novartis
10-2014
i
a Novartis company
44285-7
Keep out of the reach and sight of children.
• Do not store in a refrigerator or freezer or above 25°C.
• Keep the container in the outer carton.
• Keep the container tightly closed.
• Do not use the drops after the expiry date (marked ‘Exp’) on the bottle and the carton. The expiry date refers to the last day of that month.
• Do not use eye drops which have changed colour or contain a precipitate (visible solids).