Mydriasert 0.28 Mg/5.4 Mg Ophthalmic Insert
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Mydriasert 0.28 mg/5.4 mg ophthalmic insert
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ophthalmic insert contains 0.28 mg of tropicamide and 5.4 mg of phenylephrine hydrochloride.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Ophthalmic insert.
White to yellowish-white, oblong, 4.3 mm x 2.3 mm insert.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Mydriasert is indicated:
- to obtain pre-operative mydriasis,
- or for diagnostic purposes when monotherapy is known to be insufficient.
4.2 Posology and method of administration
Restricted use to health-care professionals.
This medicine is reserved to adults.
There are no data in children and adolescents. Mydriasert is not recommended in these patients.
Posology
One ophthalmic insert per operated eye, a maximum of 2 hours before surgery or the investigative procedure (see also 5.1).
Method of administration
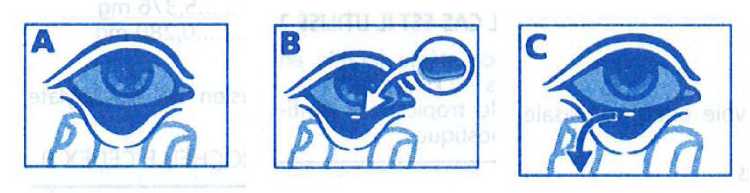
Cut the sealed edge along the dotted line, open the sachet and locate the insert.
Hold the insert with disposable sterile forceps with rounded ends provided in the packaging, making sure not to damage it.
Pull down the lower eyelid by pinching it between the thumb and index finger (A), and apply the ophthalmic insert, using the disposable sterile forceps, in the lower conjunctival sac (B).
Instructions for use
Do not leave the ophthalmic insert for more than two hours in the lower conjunctival sac. The practitioner can remove the ophthalmic insert as soon as mydriasis is deemed sufficient for the operation or procedure to be carried out, and at the latest within the next 30 minutes.
In the event of discomfort, ensure that the insert has been placed correctly at the base of the lower conjunctival sac.
Manipulate aseptically. It is recommended to avoid excessive manipulation of eyelids.
CAUTION: Removal of the ophthalmic insert
Before an operation or procedure, and as soon as the required mydriasis has been obtained, the ophthalmic insert should be removed from the lower conjunctival sac (C) by using either sterile surgical forceps, or a sterile swab or a sterile irrigation or washing solution, by lowering the lower eyelid.
Do not reuse the insert. Discard the insert after use immediately.
4.3 Contraindications
Hypersensitivity to the active substances “phenylephrine hydrochloride and tropicamide” or to any one of the excipients.
Risk of angle-closure glaucoma: Patients with closed angle glaucoma (unless previously treated with iridectomy) and patients with narrow angle prone to glaucoma precipitated by mydriatics.
4.4 Special warnings and precautions for use
Special warnings:
Because this medicinal product causes long lasting visual disturbances, the patient should be advised to be accompanied when attending the consultation (see 4.8).
Protect the eye against bright lighting after the end of intervention/consultation.
Ocular hyperemia can increase the absorption of the active ingredients contained in the insert.
Special precautions for use:
The shifting or, more rarely, the expulsion of the insert is possible. In this case, do not re use the removed insert, take a new one (see section 4.2).
Mydriasert should not be left in the conjunctival sac for more than 2 hours. In cases where Mydriasert was forgotten, local adverse reactions were observed (see section 4.8).
Because of uncommon potential irritation on conjunctiva, special care should be taken with patients suffering from severe dry eyes (use of Mydriasert in some patients may necessitate the addition of a drop of saline solution to improve insert tolerance).
All mydriatic agents may trigger an acute attack of glaucoma through the mechanical obstruction of the excretory pathways of aqueous humour in subjects presenting with a narrow iridocorneal angle.
Although not anticipated with Mydriasert due to negligible systemic passage of active ingredients, it is however reminded that phenylephrine has sympathomimetic activity that might affect patients in the event of hypertension, cardiac disorders, hyperthyroidism, atherosclerosis or prostate disorders and all subjects presenting with a contraindication to the systemic use of pressor amines.
Sportsmen and athletes should be warned that this proprietary medicinal product contains an active principle (phenylephrine) which may produce positive results to tests for prohibited substances.
The wearing of soft hydrophilic contact lenses is inadvisable during treatment.
After the insertion of Mydriasert, and if the administration of other mydriatic agents cannot be avoided, account must be taken of the doses in the insert of approximately one drop of a 10% solution of phenylephrine and approximately one drop of a 0.5% solution of tropicamide.
4.5 Interaction with other medicinal products and other forms of interaction
No specific studies interaction studies have been performed with Mydriasert.
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of phenylephrine and tropicamide in pregnant women. Animal studies are insufficient with respect to effects on pregnancy, embryonal/foetal development, parturition and postnatal development (see section 5.3).
Even though a negligible systemic uptake is expected, a low systemic exposure can not be excluded.
Therefore, Mydriasert should not be used during pregnancy unless necessary.
Lactation
No data are available concerning the passage of phenylephrine or tropicamide into breast milk. However, phenylephrine is poorly absorbed orally, implying that absorption by the infant would be negligible. On the other hand, infants may be very sensitive to anticholinergics, and despite the expected negligible systemic exposure, tropicamide is therefore not recommended during breast feeding.
Therefore, Mydriasert should not be used during breast feeding.
4.7 Effects on ability to drive and use machines
Mydriasert has major influence on the ability to drive and use machines.
Patients should be warned of the risks related to mydriatic and cycloplegic agents, which may cause visual disturbances like dizziness, drowsiness and impaired concentration: application of the Mydriasert ophthalmic insert causes disabling mydriasis for several hours; consequently, after application, the patient should be advised not to drive and/or use machines while the visual disturbances persist and/or not to perform other hazardous activities.
4.8 Undesirable effects
The following transient effects have been reported during clinical studies:
Eye disorders
Common (> 1/100):
- stinging,
- blurred vision,
- visual discomfort
Uncommon (> 1/1000, < 1/100):
- tearing, irritation,
- disabling mydriasis because of prolonged pupil dilation, photophobia,
- superficial punctuate keratitis.
Rare (< 1/1000):
- blepharitis,
- conjunctivitis,
- risk of angle-closure glaucoma, intraocular hypertension.
Very rare cases of corneal ulcer and corneal oedema were observed due to forgotten insert.
Although administered via the topical route, the mydriatic agents contained in this insert may cause the following systemic effects which must be taken into account:
-elevation of blood pressure, tachycardia,
-very rarely, major accidents such as cardiac arrhythmia,
-tremor, pallor, headaches, dry mouth.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme.
Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
Although unlikely due to single administration of Mydriasert (for either pre-operative or diagnostic purposes), a risk of overdose may nevertheless occur in the event of the additional instillation of mydriatic eyedrops.
Symptoms of a phenylephrine overdose include extreme tiredness, sweating, dizziness, a slow heartbeat, and coma.
Because severe toxic reaction to phenylephrine is of rapid onset and short duration, treatment is primarily supportive. Prompt injection of a rapidly acting alpha-adrenergic blocking agent such as phentolamine (dose 2 to 5 mg i.v.) has been recommended.
Symptoms of tropicamide ophthalmic overdoses include headache, fast heartbeat, dry mouth and skin, unusual drowsiness, and flushing.
Systemic effects from tropicamide are not expected. Should an overdose occur causing local effects, e.g. sustained mydriasis, pilocarpine or 0.25% w/v physostigmine should be applied.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: MYDRIATICS and CYCLOPLEGICS, Tropicamide combinations.
ATC code: S01F A56
Mydriasert is an ophthalmic insert which combines two synthetic mydriatic agents (phenylephrine, alpha sympathomimetic, and tropicamide, anticholinergic).
Clinical trials have shown a time to reach a stable and sufficient mydriasis between 45 and 90 min. The maximal mydriasis (pupil diameter of 9 mm) was reached in 90 to 120 minutes.
The mydriasis, when reached, lasted at least 60 minutes.
The recovery of the pupil reflex was seen at 90 minutes at the average.
5.2 Pharmacokinetic properties
After application of an insert for 2 hours in 138 patients scheduled for cataract surgery, the concentrations of the active ingredients assayed in aqueous humour were very low: 1.9±3.4 pg/ml for phenylephrine and 0.85±2.06 pg/ml for tropicamide. The cumulative quantities of the active ingredients released in 2 hours by the insert represent less than 40% of the doses contained in the insert.
In the same conditions, the plasma levels of phenylephrine measured during 6 hours in healthy volunteers were not detectable (< 0.5 ng/ml).
5.3 Preclinical safety data
Safety pharmacology, genotoxicity and conventional reproductive studies have not been conducted with phenylephrine, tropicamide or the fixed combination.
In rats, administration of phenylephrine (12.5 mg/kg, s.c.) resulted in reduced uterine blood flow (86.8% reduction in about 15 minutes), thereby exhibiting foetotoxic and co-teratogenic properties.
A 14-day local tolerance study was conducted in the rabbit, with insertion during 6 hours daily. This study demonstrated a mild irritating effect of the conjunctiva at the site of application.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Ammonio methacrylate copolymer (Type A) Polyacrylate dispersion 30%
Glycerol dibehenate Ethylcellulose.
6.2 Incompatibilities
Not applicable.
Shelf life
6.3
18 months.
After first opening of the sachet: Use immediately. After first use: Discard the used insert immediately.
6.4. Special precautions for storage
Do not store above 25°C.
6.5 Nature and contents of container
Ophthalmic insert in a sachet (Paper/PE/Aluminium/PE) and disposable sterile forceps in a sachet (Paper/PE/Aluminium/PE).
Box of 1, 10, 20, 50 and 100 inserts together with respectively 1, 10, 20, 50 and 100 forceps.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Cut the sealed edge along the dotted line, open the sachet and locate the insert.
Hold the insert with disposable sterile forceps with rounded ends provided in the packaging, making sure not to damage it; place it at the base of the lower conjunctival sac, having pulled down the lower eyelid with the thumb and index finger.
For single use only.
Use immediately after first opening the sachet.
Discard the used insert immediately.
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Laboratories Thea
12 Rue Louis-Bleriot Z.I. DU Brezet Clermont-Ferrand Cedex 2 F-63017 France
8 MARKETING AUTHORISATION NUMBER(S)
PL 20162/0011
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
08/12/2009
10 DATE OF REVISION OF THE TEXT
29/05/2015