Nicotinell Liquorice 2mg Medicated Chewing Gum
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Nicotinell Liquorice 2 mg, medicated chewing-gums
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each piece of medicated chewing-gum contains:
Active substance: 2 mg nicotine (as 10 mg nicotine - polacrilin (1:4)).
Excipient with known effect: sorbitol ( 0.19g), sodium (11.50 mg) and butylhydroxytoluene (E321)
For a full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Medicated chewing-gum.
Each piece of coated medicated chewing-gum is off-white in colour and rectangular in shape.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Relief of nicotine withdrawal symptoms, in nicotine dependency as an aid to smoking cessation.
Patient counselling and support normally improve the success rate.
4.2 Posology and method of administration Posology
Adults and elderly
Users should stop smoking completely during treatment with Nicotinell medicated chewing-gum.
The 2 mg medicated chewing-gum is not recommended for smokers with a strong or very strong nicotine dependency.
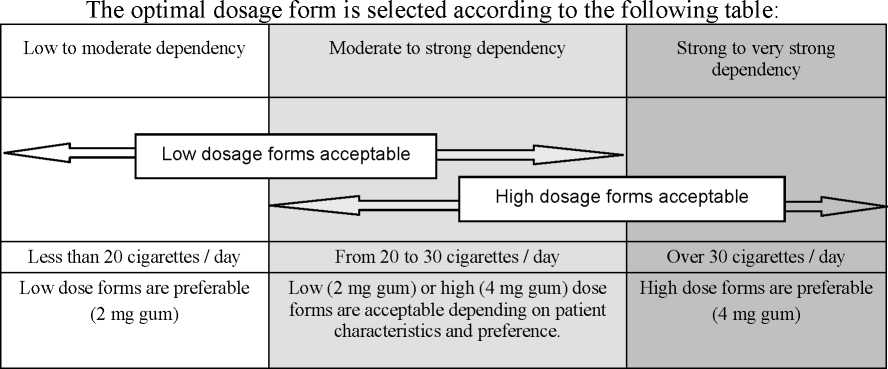
If an adverse event occurs with the use of the high dose form (4 mg medicated chewing-gum), use of the low dose form (2 mg medicated chewing-gum) should be considered.
The initial dosage should be individualised on the basis of the patients nicotine dependence.
One piece of Nicotinell medicated chewing-gum to be chewed when the user feels the urge to smoke.
If Nicotinell 2 mg medicated chewing-gum is selected, normally use 8-12 pieces per day, up to a maximum of 25 pieces per day.
The characteristics of medicated chewing-gum as a pharmaceutical form are such that individually different nicotine levels can result in the blood. Therefore, dosage frequency should be adjusted according to individual requirements within the stated maximum limit.
The treatment duration is individual. Normally, treatment should continue for at least 3 months.
After 3 months, the users should gradually reduce the number of pieces chewed each day until they have stopped using the product.
Treatment should be discontinued when the dose has been reduced to 1-2 pieces of medicated chewing-gum per day. Use of nicotine medicinal products like Nicotinell medicated chewing-gum beyond 6 months is generally not recommended. Some ex-smokers may need treatment with the medicated chewing-gum for longer to avoid returning to smoking. Patients who have been using oral nicotine replacement therapy beyond 9 months are advised to seek additional help and information from health care professionals.
Counselling may help smokers to quit.
Paediatric Population
Nicotinell medicated chewing-gum should not be used by people under 18 years of age without recommendation from a physician. There is no experience in treating adolescents under the age of 18 years with Nicotinell medicated chewing-gum.
Method of administration
1. One piece of medicated chewing-gum should be chewed until the taste becomes strong.
2. The medicated chewing-gum should be rested between the gum and cheek.
3. When the taste fades, chewing should commence again.
4. The chewing routine should be repeated for 30 minutes.
Concomitant use of acidic beverages such as coffee or soda may decrease the buccal absorption of nicotine. Acidic beverages should be avoided for 15 minutes prior to chewing the medicated chewing-gum.
4.3 Contraindications
Hypersensitivity to nicotine, liquorice or to any of the excipients listed in section 6.1.
Nicotinell medicated chewing-gum should not be used by non-smokers.
4.4 Special warnings and precautions for use
Dependent smokers with a recent myocardial infarction, unstable or worsening angina including Prinzmetal’s angina, severe cardiac arrhythmias, uncontrolled hypertensions or recent cerebrovascular accident should be encouraged to stop smoking with non-pharmacological interventions (such as counselling). If this fails, Nicotinell medicated chewing-gums may be considered but as data on safety in this patient group are limited, initiation should only be under close medical supervision.
Nicotinell medicated chewing-gums should be used with caution in patients with hypertension, stable angina pectoris, cerebrovascular disease, occlusive peripheral arterial disease, heart failure, diabetes mellitus, hyperthyroidism or pheochromocytoma and severe hepatic and/or renal impairment.
Patients should initially be encouraged to stop smoking with nonpharmacological interventions (such as counselling).
Swallowed nicotine may exacerbate symptoms in subjects suffering from active oesophagitis, oral or pharyngeal inflammation, gastritis or peptic ulcer.
Doses of nicotine that are tolerated by adult smokers during treatment may produce severe symptoms of poisoning in small children and may prove fatal (please see Section 4.9).
People having problems with the joint of the jawbone and denture wearers may experience difficulty in chewing the medicated chewing-gum. In this case, it is recommended that they use a different pharmaceutical form of nicotine replacement therapy.
Special warnings about excipients
This medicine contains low dose of extractum glycyrrhizae (liquorice) as a flavour. High and prolonged intake of liquorice may lead to mineralocorticoid effects (pseudoaldosteronism) in the form of electrolyte imbalance (sodium retention and potassium loss) accompanied by hypertension, oedema and suppression of the renin-angiotensin-aldosterone system.
However, individual tolerance varies widely and regular intake of even low amount of liquorice may cause pseudoaldosteronism in the most sensitive individuals (liquorice susceptible individuals). The possible mineralocorticoid effects due to liquorice have to be taken into consideration in liquorice susceptible patients with cardiovascular diseases and hypertension. Where use of nicotine replacement therapy is recommended the use of other flavoured nicotine medicated chewing-gums (e.g. fruit, mint or classic) may be considered.
Because Nicotinell Liquorice medicated chewing-gums contain sorbitol: Patients with rare hereditary conditions of fructose intolerance should not take this medicine.
Nicotinell Liquorice 2 mg medicated chewing-gum contains sweeteners, including sorbitol (E420) 0.19 g per medicated chewing-gum, a source of 0.04 g fructose. Calorific value 0.9 kcal/piece of medicated chewing-gum.
Nicotinell Liquorice 2 mg medicated chewing-gum contains sodium 11.50 mg per piece.
The gum base contains butylhydroxytoluene (E321) which may cause local irritation to mucous membranes.
4.5 Interaction with other medicinal products and other forms of interaction
Drug Interactions: No information is available on interactions between Nicotinell medicated chewing-gum and other medicinal products.
Smoking Cessation: Smoking but not nicotine is associated with increased CYP1A2 activity. After stopping smoking there may be reduced clearance of substrates for this enzyme and increased plasma levels of some medicinal products of potential clinical importance because of their narrow therapeutic window e.g. theophylline, tacrine, olanzapine and clozapine.
The plasma concentrations of other active substances metabolised by CYP1A2 e.g. caffeine, paracetamol, phenazone, phenylbutazone, pentazocine, lidocaine, benzodiazepines, warfarin, oestrogen and vitamin B12 may also increase. However the clinical significance of this effect for these active substances is unknown.
Smoking may lead to reduced analgesic effects of propoxyphene, reduced diuretic response to furosemide (frusemide), reduced effect of propranolol on blood pressure and heart rate and reduced responder rates in ulcer healing with H2-antagonists.
Smoking and nicotine may raise the blood levels of cortisol and catecholamines, i.e. may lead to a reduced effect of nifedipine or adrenergic antagonists and to an increased effect of adrenergic agonists.
Increased subcutaneous absorption of insulin which occurs upon smoking cessation may necessitate a reduction in insulin dose.
4.6 Fertility, Pregnancy and lactation
There is no adequate data from the use of preparations containing glycyrrhizin in pregnant and lactating women. Nicotinell Liquorice gum should therefore not be used during pregnancy and lactation. Where use of nicotine replacement therapy is recommended the use of other flavoured nicotine medicated chewing-gums (e.g. fruit, mint or classic) may be considered.
4.7 Effects on ability to drive and use machines
There is no evidence of any risks associated with driving or operating machinery when the medicated chewing-gum is used following the recommended dose. Nevertheless one should take into consideration that smoking cessation can cause behavioural changes.
4.8 Undesirable effects
Nicotinell medicated chewing-gum can cause adverse reactions similar to those associated with nicotine administered by smoking. These can be attributed to the pharmacological effects of nicotine, which are dose-dependent. Non dose-dependent adverse reactions are as follows: jaw muscle ache, erythema, urticaria, hypersensitivity, angioneurotic oedema and anaphylactic reactions.
Most of the side effects which are reported by patients occur generally during the first
3-4 weeks after initiation of therapy.
Nicotine from gums may sometimes cause a slight irritation of the throat and increase salivation at the start of the treatment.
Excessive swallowing of nicotine which is released in the saliva may, at first, cause hiccups. Those who are prone to indigestion may suffer initially from minor degrees of dyspepsia or heartburn; slower chewing will usually overcome this problem.
Excessive consumption of nicotine gums by subjects who have not been in the habit of inhaling tobacco smoke, could possibly lead to nausea, faintness and headache.
Increased frequency of aphthous ulcer may occur after abstinence from smoking.
The medicated chewing-gum may stick to and in rare cases damage dentures and dental appliances.
Adverse reactions are listed below, by system organ class and frequency. Frequencies are defined as: very common (>1/10), common (>1/100 to <1/10), uncommon (>1/1,000 to <1/100), rare (>1/10,000, <1/1,000) or very rare (<1/10,000).
Nervous system disorders:
Common: headache, dizziness Gastrointestinal disorders:
Common: hiccups, gastric symptoms e.g. nausea, flatulence, vomiting, dyspepsia, salivary hypersecretion, stomatitis, oral pain, or pharyngolaryngeal pain
Musculoskeletal, connective and bone disorders:
Common: jaw muscle ache
Cardiac disorders:
Uncommon: palpitations Rare: atrial arrhythmia
Skin and subcutaneous tissue disorders:
Uncommon: erythema, urticaria
Immune system disorders:
Rare: hypersensitivity, angioneurotic oedema and anaphylactic reactions
Certain symptoms which have been reported such as dizziness, headache and insomnia may be ascribed to withdrawal symptoms in connection with smoking cessation and may be due to insufficient administration of nicotine.
Cold sores may develop in connection with smoking cessation, but any relation with the nicotine treatment is unclear.
The patient may still experience nicotine dependence after smoking cessation. Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra. gov.ukyellowcard.
4.9 Overdose
In overdose, symptoms corresponding to heavy smoking may be seen.
The acute lethal oral dose of nicotine is about 0.5 - 0.75 mg per kg bodyweight, corresponding in an adult to 40 - 60 mg. Even small quantities of nicotine are dangerous in children, and may result in severe symptoms of poisoning which may prove fatal. If poisoning is suspected in a child, a doctor must be consulted immediately.
Overdose with Nicotinell medicated chewing-gum may only occur if many pieces are chewed simultaneously. Nicotine toxicity after ingestion will most likely be minimised as a result of early nausea and vomiting that occur following excessive nicotine exposure. Risk of poisoning by swallowing the medicated chewing-gum is small. Since the release of nicotine from the medicated chewing-gum is slow, very little nicotine is absorbed from the stomach and intestine, and if any is, it will be inactivated in the liver.
General symptoms of nicotine poisoning include: weakness, perspiration, salivation, dizziness, throat burn, nausea, vomiting, diarrhoea, abdominal pain, hearing and visual disturbances, headache, tachycardia and cardiac arrhythmia, dyspnoea, prostration, circulatory collapse, coma and terminal convulsions.
Prolonged overuse of large dose of Nicotinell Liquorice medicated chewing-gum, may cause in rare cases of liquorice susceptible individuals, reversible pseudoaldosteronism (see section 4.4).
Treatment of overdose
Treatment of overdose should be immediate as symptoms may develop rapidly. Emesis is usually spontaneous. Administration of oral activated charcoal and gastric lavage should be considered as soon as possible and within 1 hour of ingestion. Monitor vital signs and treat symptomatically.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC Code: N07B A01
Pharmacotherapeutic group: Drugs used in nicotine dependence
Nicotine, the primary alkaloid in tobacco products and a naturally occurring autonomous substance, is a nicotine receptor agonist in the peripheral and central nervous systems and has pronounced CNS and cardiovascular effects. On consumption of tobacco products, nicotine has proven to be addictive, resulting in craving and other withdrawal symptoms when administration is stopped. This craving and these withdrawal symptoms include a strong urge to smoke, dysphoria, insomnia, irritability, frustration or anger, anxiety, concentration difficulties agitation and increased appetite or weight gain. The medicated chewing-gum replaces part of the nicotine that would have been administrated via tobacco and reduces the intensity of the withdrawal symptoms and smoking urge.
5.2 Pharmacokinetic properties
When the medicated chewing-gum is chewed, nicotine is steadily released into the mouth and is rapidly absorbed through the buccal mucosa. A proportion, by the swallowing of nicotine containing saliva, reaches the stomach and intestine where it is inactivated.
The nicotine peak plasma mean concentration after a single dose of Nicotinell 2 mg medicated chewing-gum is approximately 6.4 nanograms per ml (after 45 minutes) (average plasma concentration of nicotine when smoking a cigarette is 15-30 nanograms per ml).
Nicotine is eliminated mainly via hepatic metabolism; small amounts of nicotine are eliminated in unchanged form via the kidneys. The plasma halflife is approximately three hours. Nicotine crosses the blood-brain barrier, the placenta and is detectable in breast milk.
5.3 Preclinical safety data
Nicotine was positive in some in vitro genotoxicity tests but there are also negative results with the same test systems. Nicotine was negative in standard in-vivo tests.
Animal experiments have shown that nicotine induces post-implantation loss and reduces the growth of foetuses.
The results of carcinogenicity assays did not provide any clear evidence of a tumorigenic effect of nicotine.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Gum base (containing butylhydroxytoluene (E321)) Calcium carbonate Sorbitol (E420)
Sodium carbonate anhydrous Sodium hydrogen carbonate Polacrilin Glycerol (E422)
Purified water Star anise oil
Extractum glycyrrhizae soluble Levomenthol
Eucalyptus oil Saccharin Sodium saccharin Acesulfame potassium Xylitol (E967)
Mannitol (E421)
Gelatin
Ttitanium dioxide (E171)
Carnauba wax
Talc
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
30 months
6.4 Special precautions for storage
Do not store above 25°C.
6.5 Nature and contents of container
The medicated chewing-gum is packed in PVC/PVdC/aluminium blisters each containing either 2 or 12 pieces of medicated chewing-gum. The blisters are packed in boxes containing 2, 12, 24, 36, 48, 60, 72, 84, 96, 120 and 204 pieces of medicated chewing-gum. Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Used Nicotinell medicated chewing-gum should be disposed of with care.
7 MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Consumer Healthcare (UK) Trading Limited,
980 Great West Road Brentford Middlesex TW8 9GS
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 44673/0137
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorization: 22/08/2000 Date of last renewal: 25/11/2011
10 DATE OF REVISION OF THE TEXT
02/08/2016