Olopatadine 1mg/Ml Eye Drops Solution
150mm
Package leaflet: Information for the user
Olopatadine lmg/ml eye drops, solution
Olopatadine



Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed fijr you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet See section 4. What ism this leaflet:
1. What Olopatadine is and what it is used for
2. What you need to know before you take Olopatadine
3. How to take Olopatadine
4. Possible side effects
5. How to store Olopatadine
1. What Olopatadine is and what it is used for
Olopatadine is used for the treatment of signs and symptoms of seasonal allergic conjunctivitis.
Allergic conjunctivitis: Some materials (allergens) like pollens, house dust or animal fur may cause allergic reactions resulting in itching, redness, as well as swelling of the surface of your eye. Olopatadine is a medicine for treatment of allergic conditions of the eye. It works by reducing the intensity of the allergic reaction
2. What you need to know before you take Olopatadine
Do not use Olopatadine:
• If you are allergic to olopatadine or any of the other ingredients of this medicine(listed in section 6).
• In children under the age of 3 years.
Warnings and precautions
Talk to your doctor or pharmacist before using Olopatadine
You should remove contact lenses that are in your eyes before using Olopatadine.
Other medicines and Olopatadine
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. If you are using other eye drops or eye ointment medicines, leave at least S minutes between each medicine. Eye ointments should be administered last
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may he pregnant or are planning to have a baby, ask your doctor or pharmacist for advise before using this medicine. You should not use Olopatadine if you are breast-feeding, ask your doctor for advise before using this medicine. Driving and using machines
You may find that your vision is blurred for a time just after you use Olopatadine. Do not drive or use machines until this has worn off. Olopatadine contains benzalkonium chloride Benzalkonium chloride may cause eye irritation and is known to discolour soft contact lenses, therefore contact with soft contact lenses should be avoided. If you wear contact lens you should remove contact lenses prior to application and wait at least IS minutes before putting your lenses back in.
3. How to take Olopatadine
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure. The recommended dose is one drop in the eye or eyes, twice a day - morning and evening. Use this much unless your doctor tells you to do differently. Only use Olopatadine in both eyes if your doctor told you to. Use it for as long as your doctor told you to. Only use Olopatadine as an eye drop.
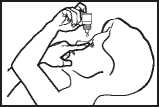
Instructions on using Olopatadine
• Take the bottle and twist off the cap.
• Hold the bottle, pointing down, between your thumb and middle finger.
• Tilt your head back. Pull down your eyelid with a clean finger, until there is a‘pocket between the eyelid and your eye. The drop will go in here.
• Bring the bottle top close to the eye. Use the mirror if it helps.
• Don’t touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops left in the bottle.
• Gently press on the base of the bottle to release one drop of Olopatadine at a time.
• Don’t squeeze the bottle, it is designed so that just a gentle press on the bottom is needed
200mm
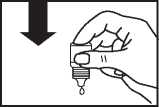

Do not use this medicine after the expiry date which is stated on the label (EXP). The expiry date refers to the last day of that month.
• If you use drops in both eyes, repeat the steps for your other eye.
• Put the bottle cap back on firmly immediately alter use.
• Use 15) one bottle before opening the next bottle.
• If a drop misses your eye, try again.
If you take more Olopatadine than you should
Rinse it all out with warm water. Don’t put in any more drops until it’s
time for your next regular dose.
If you forget to take Olopatadine
Use a single drop as soon as you remember, and then go back to your regular routine. Do not use a double dose to make up for the one missed. If you stop using Olopatadine
Do not stop using this medicine without first speaking to your doctor. If you have any further questions on the use of this medicine, ask your doctor or pharmacist

Like all medicines, this medicine can cause side effects, although not everybody gets them. They are usually mild to moderate.
Common (mm effect mom than 1 in 10 people)
eye pain or swelling, eye irritation, dry eye, abnormal eye sensation, eye
discomfort
General side effects: headache, fatigue, dry nose, bad taste.
Uncommon (max effect up to 1 in 100 people)
Effects in the eye: blurred, reduced, or abnormal vision, corneal disorder, eye surface inflammation with or without surface damage, inflammation or infection of the conjunctiva, eye discharge, sensitivity to light, increased tear production, itchy eye, redness of the eye, eyelid abnormality, itching, redness, swelling, or crusting of the eyelid.
General side effects: abnormal or decreased sensation, dizziness, runny nose, dry skin, skin inflammation
Not known: frequency cannot be estimated firm the available data Effects in the eye: eye swelling, corneal swelling, change in pupil size
General side effects: shortness of breath, increased allergic symptoms, fecial swelling, drowsiness, generalized weakness, nausea, vomiting, sinus infection, skin redness and itching.
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist
Repotting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
You must throw away the bottle four weeks after you first opened it, to prevent infections, and use a new bottle. Write down the date you opened it in the space on each bottle label and box.
Do not use this medicine if you notice a change in colour or smell.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.

What Olopatadine contains
- The active substance is olopatadine lmg/ml (as hydrochloride)
- The other ingredients are benzalkonium chloride, hydrochloride acid (E507) (for pH adjustment), disodium phosphate dodecahydrate (E339); sodium chloride, sodium hydroxide; water for injections.
What Olopatadine looks like and contents of the pack
Clear to almost clear colourless solution, practically free from particles.
5 ml opaque low density polyethylene (LDPE) bottle with LDPE dropper and high density polyethylene (HDPE) tamper-proof screw-cap.
Packsizes:
1 x 5 ml (1 bottle, which contains 5 ml of solution).
Marketing Authorisation Holder and Manufacturer: PharmaMatchB.V
Address: Egelenbuig 2, Amsterdam, 1081, GK, Netherlands Postcode: 1081 Country: Netherlands
This medicinal product is authorised in the Member States of the EEA under the Mowing names:
UK: Olopatadine lmg/ml eye drops, solution
Es: Olopatadina Pharmamafch lmg/ml colirio en solution
This leaflet was last revised in October 2015
Detailed information on this medicine is available on the web site of www.mhra.gov.uk
E
E
200