Oxis 12 Turbohaler
Out of date information, search anotherNotes:
CUSTOMER:
PRODUCT: Oxis 12 turbohaler
CODE:
PRE-PRESS NO.: 02-1808 ARTWORKER: DT
DATE OF PROOF: 11/06/14 TVT CHECKED
Q.A.
APPROVED:
DATE:
CUSTOMER
APPROVED:
DATE:
PROOF HISTORY:
v.3 - -11/06/14
Leaflet Flat Size = 296 x 317 ARIAL REGULAR FONT SIZE 8 ARIAL BOLD FONT SIZE 10 BRIDGED TO TRANSTEC
UK PIL DATED MAY 2013 REPORTING OF SIDE EFFECTS
POM
Pg 4 672-4181E pgi
OXIS® 12 Turbohaler®
(formoterol fumarate)
Patient Information Leaflet
Your medicine is called by the above name but will be referred to as Oxis Turbohaler throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Oxis Turbohaler is and what it is used for
2. Before you use Oxis Turbohaler
3. How to use Oxis Turbohaler
4. Possible side effects
5. How to store Oxis Turbohaler
6. Further information
4. Possible side effects
Like all medicines, Oxis Turbohaler can cause side effects, although not everybody gets them.
If the following happens to you, stop using Oxis Turbohaler and talk to your doctor immediately:
• Bronchospasm (tightening of the muscles in the airways which causes sudden wheezing) after inhaling your medicine. This happens very rarely, affecting less than 1 in 10,000 people.
Other possible side effects:
Common (affects less than 1 in 10 people)
• Palpitations (awareness of your heart beating), trembling or shaking. If these effects occur, they are usually mild and usually disappear as you continue to use Oxis Turbohaler.
• Headache.
Uncommon (affects less than 1 in 100 people)
• Feeling restless or agitated.
• Disturbed sleep.
• Fast heart beat.
• Muscle cramps.
Rare (affects less than 1 in 1,000 people)
• Uneven heart beat.
• Nausea (feeling sick).
• Low level of potassium in your blood.
• Allergic reactions such as rash, itching and bronchospasm.
Very rare (affects less than 1 in 10,000 people)
• Chest pain or tightness in the chest (angina pectoris).
• An increase in the amount of sugar (glucose) in your blood.
• Taste changes, such as an unpleasant taste in the mouth.
• Changes in your blood pressure.
• Feeling dizzy.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Oxis Turbohaler
Do not store your Turbohaler above 30°C. Replace the cover of your Turbohaler properly after use.
Do not use after the expiry date printed on the turbohaler or carton label.
KEEP ALL MEDICINES OUT OF THE SIGHT AND REACH OF CHILDREN.
Do not use a damaged Turbohaler.
If your doctor tells you to stop using the Turbohaler, please take it back to the pharmacist for safe disposal. Only keep the Turbohaler if your doctor tells you to.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer required. This will help to protect the environment.
If the Turbohaler fails to work properly or shows visible signs of deterioration, you should seek the advice of your pharmacist before using it.
6. Further information
The active substance is formoterol fumarate, dihydrate. Each dose contains 12 micrograms of formoterol fumarate dihydrate of which you can inhale 9 micrograms.
Oxis 12 Turbohaler is a metered dose dry powder inhaler with a dark-greenish blue turning grip and white cover. On the bottom of the turning grip, Braille '5' is embossed.
The contents are white to off white rounded granules.
Oxis Turbohaler also contains lactose monohydrate (which contains milk proteins).
Oxis Turbohaler contains 60 actuations or puffs.
PL No: 6464/1899 Oxis 12 Turbohaler
1. What Oxis Turbohaler is and what it is used for
Oxis Turbohaler is an inhaler. It contains a medicine called formoterol. This belongs to a group of medicines called ‘long-acting beta-agonists' or‘bronchodilators'.
It works by relaxing the muscles in your airways. This helps you to breathe more easily. It starts to work within 1 to 3 minutes and the effects last up to 12 hours.
Your doctor has prescribed this medicine to treat asthma or chronic obstructive pulmonary disease (COPD). Asthma
For asthma, your doctor will prescribe two asthma inhalers: Oxis Turbohaler and a separate ‘corticosteroid' inhaler. These should be used together.
• Oxis Turbohaler is used to help prevent asthma symptoms from happening.
• Some people also use Oxis Turbohaler when they need extra doses for relief of asthma symptoms, to make it easier to breathe again.
• Oxis Turbohaler can also be used before exercise to prevent asthma symptoms caused by exercise.
Chronic obstructive pulmonary disease (COPD)
Oxis Turbohaler can also be used to treat the symptoms of COPD in adults. COPD is a long-term disease of the airways in the lungs, which is often caused by cigarette smoking.
2. Before you use Oxis Turbohaler
Do not use Oxis Turbohaler if:
• You are allergic (hypersensitive) to formoterol, or the other ingredient, lactose (which contains small amounts of milk proteins).
Take special care with Oxis Turbohaler
Before you use Oxis Turbohaler, tell your doctor or pharmacist if:
• You are diabetic. You may need some additional blood sugar tests while you are using Oxis Turbohaler.
• You have high blood pressure or you have ever had a heart problem.
• You have problems with your thyroid gland.
• You have low levels of potassium in your blood. Your doctor may take blood samples to check the levels of potassium in your blood.
• You have severe liver problems such as liver cirrhosis.
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Oxis Turbohaler.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines that you buy without a prescription and herbal medicines. This is because Oxis Turbohaler can affect the way some medicines work and some medicines can have an effect on Oxis Turbohaler.
This product is manufactured by AstraZeneca AB, Sodertalje, Sweden and is procured from within the EU and repackaged by the Product Licence holder:
Waymade plc, Miles Gray Road, Basildon, Essex SS14 3FR
Leaflet revision and issue date (Ref.) 09.04.2014
Oxis and Turbohaler are registered trademarks of the AstraZeneca AB.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
• Beta-blocker medicines (such as atenolol or propranolol for high blood pressure), including eyedrops (such as timolol for glaucoma).
• Medicines for a fast or uneven heart beat (such as quinidine).
• Medicines like digoxin, often used to treat heart failure.
• Diuretics, also known as 'water tablets' (such as furosemide). These are used to treat high blood pressure.
• Steroid medicines that you take by mouth (such as prednisolone).
• Xanthine medicines (such as theophylline or aminophylline). These are often used to treat asthma.
• Erythromycin (used to treat infections).
• Anti-histamines (such as terfenadine).
• Other substances that dilate the airways (bronchodilators, such as salbutamol).
• Ephedrine (used to treat asthma or as a decongestant).
• Tricyclic anti-depressants (such as amitriptyline).
If any of the above applies to you, or if you are not sure, talk to your doctor or pharmacist before using Oxis Turbohaler.
Also tell your doctor or pharmacist if you are going to have a general anaesthetic for an operation or for dental work.
The addition of anticholinergics (such as tiotropium or ipratropium bromide) to the treatment with Oxis Turbohaler, can help to open your airways even more.
Colour
Swatch

WARNING! WE CANNOT ACCEPT RESPONSIBILITY FOR ANY ERRORS IN THIS PROOF AFTER APPROVAL. THE ARTWORK RECEIVED HAS BEEN SIGNIFICANTLY ADJUSTED, REVISED OR RESET BY US FROM DISK OR HARD COPY. WHILST WE TAKE EXTREME CARE AT ALL TIMES TO ENSURE ACCURACY, THE FINAL RESPONSIBILITY MUST BETAKEN BY OUR CUSTOMER. IF YOU SIGN THIS PROOF YOU ARE SIGNIFYING FULL APPROVAL OF DESIGN AND TEXT.
WARNING! THE COLOURS SHOWN ON THIS PROOF ARE FOR GENERAL REPRESENTATION PURPOSES ONLY. THEY ARE NOT ACCURATE AND MUST NOT BE USED AS A COLOUR MATCH FOR THE FINISHED JOB. PLEASE REFER TO THE PANTONE COLOUR GUIDES FOR ACCURATE COLOUR REFERENCES.
CUSTOMER: Waymade PRODUCT Oxis 12 turbohaler CODE: 672-4181E 6464/1899 E
PRE-PRESS NO.: 02-1808 ARTWORKER: DT
DATE OF PROOF: 11/06/14 TVT CHECKED
Q.A.
APPROVED:
DATE:
CUSTOMER
APPROVED:
DATE:
PROOF HISTORY:
v.3 - waymade -11/06/14
Leaflet Flat Size = 296 x 317 ARIAL REGULAR FONT SIZE 8 ARIAL BOLD FONT SIZE 10 BRIDGED TO
TRANSTEC 6464/2327 2328 2329
UK PIL DATED MAY 2013 REPORTING OF SIDE EFFECTS
Pg2
Pg 3
Pregnancy and breast-feeding
• If you are pregnant, or planning to get pregnant, talk to your doctor before using Oxis Turbohaler -do not use Oxis Turbohaler unless your doctor tells you to.
• If you get pregnant while using Oxis Turbohaler, do not stop using Oxis Turbohaler but talk to your doctor immediately.
• If you are breast-feeding, talk to your doctor before using Oxis Turbohaler.
Driving and using machines
Oxis Turbohaler is not likely to affect you being able to drive or use any tools or machines.
Important information about some of the ingredients of Oxis Turbohaler
Oxis Turbohaler contains lactose, which is a type of sugar. If you have been told by your doctor that you have an intolerance to some sugars, talk to your doctor before using this medicine. The amount of lactose in this medicine does not normally cause problems in people who are lactose intolerant.
Preparing your new Oxis Turbohaler
Before using your new Oxis Turbohaler for the first time, you need to prepare it for use as follows:
• Unscrew the white cover and lift it off. You may hear a rattling sound.
• Hold your Oxis Turbohaler upright with the turquoise grip at the bottom.
• Turn the turquoise grip as far as it will go in one direction. Then turn it as far as it will go in the other direction (it does not matter which way you turn first).
You should hear a click sound.
• Do this again, turning the turquoise grip in both directions.
• Your Oxis Turbohaler is now ready for use.
Mouthpiece Indicator —
The excipient lactose contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Oxis Turbohaler
• Always use Oxis Turbohaler exactly as your doctor, nurse or pharmacist has told you. Ask one of them for advice if you are not sure.
• Do not increase the dose of Oxis Turbohaler prescribed by your doctor without talking to your doctor first.
• If you are using Oxis Turbohaler regularly for asthma or COPD you should continue to use your medicine, even if you have no symptoms.
Important information about your asthma or COPD symptoms
If you feel you are getting breathless or wheezy while using Oxis Turbohaler, you should continue to use Oxis
Turbohaler but go to see your doctor as soon as possible, as you may need additional treatment.
Contact your doctor immediately if:
• Your breathing is getting worse or you often wake up at night with asthma.
• You start getting chest tightness.
• You are not getting relief from your current dose.
• You need to take more than your usual dose for more than two days in a week.
• You need to use your Turbohaler more often than usual before exercise.
These signs could mean that your asthma or COPD is not being properly controlled and you may need
different or additional treatment immediately.
Asthma
Oxis Turbohaler should not be used in children under 6 years of age.
Adults (18 years and above)
• The usual dose is 1 inhalation, once or twice a day.
• Your doctor may increase this to 2 inhalations, once or twice a day.
• Some people also use Oxis Turbohaler as a 'reliever inhaler'. If you get asthma symptoms, the usual dose is 1 inhalation when they happen.
• A total daily dose of more than 4 inhalations is not normally needed. This includes the inhalations that you take every day, when you get asthma symptoms and before exercise. However, your doctor may allow you to take up to 6 inhalations a day. Do not use more than 6 inhalations in total in 24 hours.
• Do not take more than 3 inhalations at any one time.
Children and adolescents (6 to 17 years)
• The usual dose is 1 inhalation once or twice a day.
• Some children also use Oxis Turbohaler as a ‘reliever inhaler'. If your child gets asthma symptoms, the usual dose is 1 inhalation when they happen.
• A total daily dose of more than 2 inhalations is not normally needed.
This includes the inhalations that your child takes every day, when they get asthma symptoms and before exercise. However, your doctor may allow your child to take up to 4 inhalations a day. Your child should not use more than 4 inhalations in total in 24 hours.
• Your child should not have more than 1 inhalation at any one time.
How to take an inhalation
Every time you need to take an inhalation, follow the instructions below. 1. Unscrew the white cover and lift it off.
2. Hold your Turbohaler upright with the turquoise grip at the bottom.
3. Do not hold the mouthpiece when you load your Turbohaler. To load your Turbohaler with a dose, turn the turquoise grip as far as it will go in one direction. Then turn it as far as it will go in the other direction (it does not matter which way you turn it first).
You should hear a click sound. Your Turbohaler is now loaded and ready to use.
It is not possible to overload your Turbohaler even if you turn the grip several times. Only load your Turbohaler when you need to use it.
Grip ■
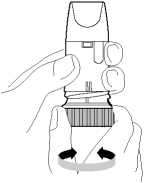
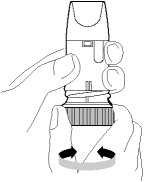
4. Hold your Turbohaler away from your mouth. Breathe out gently (as far as is comfortable) Do not breathe out through your Turbohaler.
5. Place the mouthpiece gently between your teeth. Close your lips. Breathe in as deeply and as hard as you can through your mouth. Do not chew or bite on the mouthpiece.
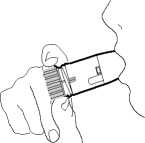
6. Remove your Turbohaler from your mouth. Then breathe out gently. The amount of medicine that is inhaled is very small. This means you may not be able to taste it after inhalation. If you have followed the instructions, you can still be confident that you have inhaled the dose and the medicine is now in your lungs.
7. If you are to take a second inhalation, repeat steps 2 to 6.
8. Replace the cover tightly after use.
Do not try to remove or twist the mouthpiece. It is fixed to your Turbohaler and must not be taken off. Do not
use your Turbohaler if it has been damaged or if the mouthpiece has come apart from your Turbohaler.
Cleaning your Turbohaler
Wipe the outside of the mouthpiece once a week with a dry tissue.
Do not use water or liquids.
Your doctor (or asthma nurse) will help you to manage your asthma. Once your asthma is well controlled your doctor may consider it appropriate to gradually reduce the dose of Oxis Turbohaler.
Asthma caused by exercise
If you or your child have asthma symptoms caused by exercise, your doctor may advise you or your child to use Oxis Turbohaler before exercise as well. Oxis Turbohaler should not be used in children under 6 years of age.
Adults (18 years and above)
• The usual dose is 1 inhalation before exercise.
• A total daily dose of more than 4 inhalations is not normally needed. This includes the inhalations that you take every day, when you get asthma symptoms and before exercise. However, your doctor may allow you to take up to 6 inhalations a day. Do not use more than 6 inhalations in total in 24 hours.
• Do not take more than 3 inhalations at any one time.
Children and adolescents (6 to 17 years)
• The usual dose is 1 inhalation before exercise.
• A total daily dose of more than 2 inhalations is not normally needed. This includes the inhalations that your child takes every day, when they get asthma symptoms and before exercise. However, your doctor may allow your child to take up to 4 inhalations a day. Your child should not use more than 4 inhalations in total in 24 hours.
• Your child should not have more than 1 inhalation at any one time.
Chronic obstructive pulmonary disease (COPD)
• Only to be used by adults (aged 18 years and above).
• The usual dose is 1 inhalation once or twice a day.
• Your doctor may advise you to take extra doses for relief of your COPD symptoms.
• You should not have more than 4 inhalations a day.
• Do not take more than 2 inhalations at any one time.
Pg 3 ^
When to start using a new Turbohaler
• The Turbohaler contains 60 doses (inhalations). The dose indicator tells you how many doses are left in the Turbohaler.
• When you first see a red mark at the edge of the indicator window, there are approximately 20 doses left. When the red mark reaches the bottom of the indicator window, you must start using your new Turbohaler.

Note:
• The grip will still twist and 'click' even when your Turbohaler is empty.
• The sound that you hear as you shake your Turbohaler is produced by a drying agent and not the medicine. Therefore the sound does not tell you how much medicine is left in your Turbohaler.
If you use more Oxis Turbohaler than you should
If you use more Oxis Turbohaler than you should, contact your doctor or pharmacist for advice immediately. The following effects may happen: trembling, headache ora rapid heart beat.
If you forget to use Oxis Turbohaler
• If you forget to take a dose, take it as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
• Do not take a double dose to make up for a forgotten dose.
If you stop using Oxis Turbohaler
Do not stop using Oxis Turbohaler without talking to your doctor.
If you have any further questions on using your Turbohaler, ask your doctor, nurse or pharmacist. 672-4181E
Pg4 ^
Colour
Swatch

WARNING! WE CANNOT ACCEPT RESPONSIBILITY FOR ANY ERRORS IN THIS PROOF AFTER APPROVAL. THE ARTWORK RECEIVED HAS BEEN SIGNIFICANTLY ADJUSTED, REVISED OR RESET BY US FROM DISK OR HARD COPY. WHILST WE TAKE EXTREME CARE AT ALL TIMES TO ENSURE ACCURACY, THE FINAL RESPONSIBILITY MUST BETAKEN BY OUR CUSTOMER. IF YOU SIGN THIS PROOF YOU ARE SIGNIFYING FULL APPROVAL OF DESIGN AND TEXT.
WARNING! THE COLOURS SHOWN ON THIS PROOF ARE FOR GENERAL REPRESENTATION PURPOSES ONLY. THEY ARE NOT ACCURATE AND MUST NOT BE USED AS A COLOUR MATCH FOR THE FINISHED JOB. PLEASE REFER TO THE PANTONE COLOUR GUIDES FOR ACCURATE COLOUR REFERENCES.