Paracetamol/Ibuprofen 500mg/150mg Film-Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Paracetamol/Ibuprofen 500mg/150mg Film-coated Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains Paracetamol 500 mg and Ibuprofen 150 mg.
Excipient with known effect: Lactose monohydrate 3.81 mg For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Film coated tablet (tablet)
White, capsule shaped, film coated tablets, 19mm in length, with score-line on one side and plain on the other side.
The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Paracetamol/Ibuprofen is indicated for temporary relief of acute pain associated with: headache (not migraine), backache, dental pain, muscular pain and sore throat.
Paracetamol/Ibuprofen is indicated for fever.
4.2 Posology and method of administration
Posology
For oral administration and short term use only.
The lowest effective dose should be used for the shortest time necessary to relieve symptoms.
Paracetamol/Ibuprofen should not be used for more than 3 days.
Adults
The usual dosage is one to two tablets taken every six hours, as required, up to a maximum of eight tablets in 24 hours.
Children under18 years
This product is not recommended for children under 18 years.
Elderly
No special dosage modifications are required (see section 4.4).
The elderly are at increased risk of the serious consequences of adverse reactions. If an NSAID is considered necessary, the lowest effective dose should be used for the shortest possible duration. The patient should be monitored regularly for gastrointestinal bleeding during NSAID therapy.
Renal impairment
Caution should be taken with ibuprofen dosage in patients with renal impairment. The dosage should be assessed individually. The dose should be kept as low as possible and renal function should be monitored (see sections 4.3, 4.4 and 5.2).
|
Glomerular filtration rate |
Dose |
|
10-50 ml/min |
500 mg every 6 hours |
|
< 10 ml/min |
500 mg every 8 hours |
In patients with renal insufficiency, the paracetamol dose should be reduced:_
Hepatic impairment
Caution should be taken with ibuprofen dosage in patients with hepatic impairment. The dosage should be assessed individually and the dose should be kept as low as possible (see sections 4.3, 4.4 and 5.2).
In patients with impaired hepatic function or Gilbert’s syndrome, the dose of paracetamol must be reduced or the dosing interval prolonged.
Method of administration
This product can be taken with or without food with a full glass of water. To minimise side effects it is however recommended that patients take Paracetamol/Ibuprofen with food.
4.3 Contraindications
This product is contraindicated for use:
• in patients with known hypersensitivity reaction to paracetamol, ibuprofen, other NSAIDs or to any of the excipients listed in section 6.1.
• in patients with active alcoholism as chronic excessive alcohol ingestion may predispose patients to hepatoxicity (due to the paracetamol component).
• in patients who have experienced asthma, urticaria, or allergic-type reactions after taking acetylsalicylic acid or other NSAIDs.
• in patients with a history of, or active gastrointestinal bleeding or peptic ulceration.
• during the third trimester of pregnancy (see section 4.6).
• in patients with severe heart failure, severe hepatic failure or severe renal failure (see section 4.4.).
• in patients with cerebrovascular or other active bleeding.
• in patients with blood-clotting disorders and conditions involving an increased tendency to bleeding.
4.4 Special warnings and precautions for use
The use of Paracetamol/Ibuprofen with concomitant NSAIDs including cyclooxygenase-2 selective inhibitors and doses of acetylsalicylic acid above 75 mg daily should be avoided due to the increased risk of serious gastrointestinal adverse effects.
The concomitant use of Paracetamol/Ibuprofen with other paracetamol containing product should be avoided due to the increased risk of serious liver damage.
Undesirable effects may be minimized by using the lowest effective dose for the shortest duration necessary to control symptoms (see section 4.2 and Gastrointestinal Events and Cardiovascular Thrombotic Events below). Patients treated with NSAIDs long term should undergo regular medical supervision to monitor for adverse events.
Hepatic Impairment
The use of paracetamol at higher than recommended doses can lead to hepatotoxicity and even hepatic failure and death. Also, patients with impaired liver function or a history of liver disease, and who are on long term ibuprofen therapy or paracetamol treatment should have hepatic function monitored at regular intervals, as ibuprofen has been reported to have a minor and transient effect on liver enzymes.
Severe hepatic reactions, including jaundice and cases of fatal hepatitis, though rare, have been reported with ibuprofen as with other NSAIDs. If abnormal liver tests persist or worsen, or if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g. eosinophilia, rash, etc.), ibuprofen should be discontinued. Both active drugs have been reported to cause hepatoxicity and even hepatic failure, especially paracetamol.
Patients should be advised not to take other paracetamol containing or ibuprofen containing products concurrently.
Renal Impairment
Caution is advised in the administration of paracetamol to patients with moderate and severe renal insufficiency. For the ibuprofen component of this product - caution should be used when initiating treatment with ibuprofen in patients with dehydration or renal impairment. The two major metabolites of ibuprofen are excreted mainly in the urine and impairment of renal function may result in their accumulation. The significance of this is unknown. The use of NSAIDs may result in deterioration of renal function. The dose should be kept as low as possible and assessment of renal function should occur prior to the initiation of therapy and regularly thereafter..
Combination use of ACE inhibitors or angiotensin receptor antagonists, antiinflammatory drugs and thiazide diuretics
The use of an ACE inhibiting drug (ACE-inhibitor or angiotensin receptor antagonist), an anti-inflammatory drug (NSAID or COX-2 inhibitor) and thiazide diuretic at the same time increases the risk of renal impairment. This includes use in fixed-combination products containing more than one class of drug. Combined use of these medications should be accompanied by increased monitoring of serum creatinine, particularly at the institution of the combination. The combination of drugs from these three classes should be used with caution particularly in elderly patients or those with pre-existing renal impairment.
Elderly
No adjustment in labelled dosage is necessary for older patients who require paracetamol therapy. Those who require therapy for longer than 3 days should consult their physician for condition monitoring; however, no reduction in recommended dosage is necessary. However, caution should be taken with regard to the use of ibuprofen as it should not be taken by adults over the age of 65 without consideration of co- morbidities and co-medications because of an increased risk of adverse effects, in particular heart failure, gastrointestinal ulceration and renal impairment.
Haematological Effects
Blood dyscrasias have been rarely reported. Patients on long-term therapy with ibuprofen should have regular haematological monitoring.
Coagulation Defects
Like other NSAIDs, ibuprofen can inhibit platelet aggregation. Ibuprofen has been shown to prolong bleeding time (but within the normal range), in normal subjects. Because this prolonged bleeding effect may be exaggerated in patients with underlying haemostatic defects, products containing ibuprofen should be used with caution in persons with intrinsic coagulation defects and those on anti-coagulation therapy.
Gastrointestinal Events
Upper gastro-intestinal ulcers, gross bleeding or perforation have been described with NSAIDs. The risks increase with dose and duration of treatment, and are more common in patients over the age of 65 years. Some patients will experience dyspepsia, heartburn, nausea, stomach pain or diarrhoea.
Due to the ibuprofen component should be given with care to patients with a history of GI disease (ulcerative colitis, Chrohn’s disease) as well as in patients with porphyria and varicella.
This product should be discontinued if there is any evidence of gastrointestinal bleeding.
Cardiovascular Thrombotic Events
Observational studies have indicated that non-selective NSAIDs, such as ibuprofen, may be associated with an increased risk of serious cardiovascular events, including myocardial infarction and stroke, which may increase with dose or duration of use. The risks are described as minimal at maximum daily doses which include ibuprofen at 1200 mg, the recommended maximum dose in this product.
Patients with cardiovascular disease or cardiovascular risk factors may also be at greater risk. To minimise the potential risk of an adverse cardiovascular event in patients taking an NSAID, especially in those with cardiovascular risk factors, the lowest effective dose should be used for the shortest possible duration.
There is no consistent evidence that the concurrent use of acetylsalicylic acid mitigates the possible increased risk of serious cardiovascular thrombotic events associated with NSAIDs use.
Hypertension:
NSAIDs may lead to onset of new hypertension or worsening of pre-existing hypertension and patients taking antihypertensive medicines with NSAIDs may have an impaired anti-hypertensive response. Caution is advised when prescribing NSAIDs to patients with hypertension. Blood pressure should be monitored closely during initiation of NSAID treatment and at regular intervals thereafter.
Heart failure
Fluid retention and oedema have been observed in some patients taking NSAIDs; therefore caution is advised in patients with fluid retention or heart failure.
Severe Skin Reactions
NSAIDs may very rarely cause serious cutaneous adverse events such as exfoliative dermatitis, toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), which can be fatal and occur without warning. These serious adverse events are idiosyncratic and are independent of dose or duration of use.
Patients should be advised of the signs and symptoms of serious skin reactions and to consult their doctor at the first appearance of a skin rash or any other sign of hypersensitivity.
Pre-existing asthma
Products containing ibuprofen should not be administered to patients with acetylsalicylic acid sensitive asthma and should be used with caution in patients with pre-existing asthma.
Ophthalmological effects
Adverse ophthalmological effects have been observed with NSAIDs; accordingly, patients who develop visual disturbances during treatment with products containing ibuprofen should have an ophthalmological examination.
Respiratory disorders
In patients suffering from, or with a history of, bronchial asthma or allergic disease NSAIDs have been reported to precipitate bronchospasm.
SLE and mixed connective tissue disease
In patient with systemic lupus erythematosus (SLE) and mixed connective tissue disease disorders there may be an increased risk of aseptic meningitis (see Section 4.8).
Impaired female fertility
The use of the product may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of the product should be considered.
Potential Laboratory Test Interferences
Using current analytical systems, paracetamol does not cause interference with laboratory assays. However, there are certain methods with which the possibility of laboratory interference exists, as described below:
Urine Tests:
Paracetamol in therapeutic doses may interfere with the determination of 5-hydroxyindoleacetic acid (5HIAA), causing false-positive results. False determinations may be eliminated by avoiding paracetamol ingestion several hours before and during the collection of the urine specimen.
Masking Signs of Infection
As with other drugs of this class containing ibuprofen, by reducing fever this may mask the usual signs of infection.
Special Precautions
After long term treatment (> 3 months) of analgesics with use every second day or more frequently, headache may develop or aggravate. Headache caused by overuse of analgesics (MOH - medication-overuse headache) should not be treated by increasing the dose. In such cases the use of analgesics should be discontinued in consultation with a doctor.
In order to avoid exacerbation of disease or adrenal insufficiency, patients who have been on prolonged corticosteroid therapy should have their therapy tapered slowly rather than discontinued abruptly when products containing ibuprofen are added to the treatment program.
One film-coated tablet contains 3.81 mg of lactose, resulting in 30.48 mg of lactose per maximum recommended daily dose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
This product (like any other paracetamol and/or ibuprofen containing products) should not be taken in combination with other paracetamol and/or ibuprofen products due to increased risk of serious adverse effects.
Ibuprofen:
As with other Ibuprofen containing_products, the _following combinations with Paracetamol/Ibuprofen should be avoided:
The dicumarol group: NSAIDs may increase the effect of anticoagulants such as warfarin Experimental studies show that ibuprofen reinforces the effects of warfarin on bleeding time. NSAIDs and the dicumarol group are metabolised by the same enzyme, CYP2C9.
Anti-platelet agent: NSAIDs should not be combined with antiplatelet agents such as ticlopidine due to the additive inhibition of the platelet function (see below).
Methotrexate: NSAIDs inhibit the tubular secretion of methotrexate and some metabolic interaction with reduced clearance of methotrexate may also occur as a result. Accordingly, in high-dose treatment with methotrexate one should always avoid prescribing NSAIDs (see below).
Acetylsalicylic acid: The combination of acetylsalicylic acid and other NSAIDs should be avoided due to the increased bleeding risk.
Experimental data suggest that ibuprofen may inhibit the effect of low dose acetylsalicylic acid on platelet aggregation when they are dosed concomitantly. However, the limitations of these data and the uncertainties regarding extrapolation of ex vivo data to the clinical situation imply that no firm conclusions can be made for regular ibuprofen use, and no clinically relevant effect is considered to be likely for occasional ibuprofen use (see section 5.1).
Cardiac glycosides: NSAIDs can exacerbate heart failure, reduce glomerular filtration and increase plasma cardiac glycoside (e.g. digoxin) levels.
Mifepristone: A decrease of the efficacy of the medicinal product can theoretically occur due to the antiprostaglandin properties of non-steroidal anti-inflammatory drugs (NSAIDs) including acetylsalicylic acid. Limited evidence suggests that coadministration of NSAIDs on the day of prostaglandin administration does not adversely influence the effects of mifepristone or the prostaglandin on cervical ripening or uterine contractility and does not reduce the clinical efficacy of medical termination of pregnancy
Sulphonylureas: There are rare reports of hypoglycaemia in patients on sulphonylurea medications receiving ibuprofen.
Zidovudine: There is evidence of an increased risk of haemarthroses and haematoma in HIV(+) haemophiliacs receiving concurrent treatment with zidovudine and ibuprofen.
Quinolone antibiotics: animal data indicate that NSAIDs can increase the risk of convulsions associated with quinolone antibiotics. Patients taking NSAIDs and quinolones may have increased risk of developing convulsions.
The _following combinations with Paracetamol/Ibuprofen may require dose adjustment:
NSAIDs can reduce the effect of diuretics and other antihypertensive agents.
NSAIDs may reduce the excretion of aminoglycosides.
Children: Care should be taken during concomitant treatment with ibuprofen and aminoglycosides.
Lithium: Ibuprofen reduces the renal clearance of lithium, as a result of which serum lithium levels may rise. The combination should be avoided unless frequent checks of serum lithium can be carried out and a possible reduction in the dose of lithium made.
ACE inhibitors and angiotensin-II antagonists:
There is an increased risk of acute renal failure, usually reversible, in patients with renal impairment (e.g. dehydrated and/or elderly patients) when treatment with ACE inhibitors or angiotensin-II antagonists is given at the same time as NSAIDs, including selective cyclooxygenase-2 inhibitors. The combination should, therefore, be given with care to patients with renal impairment, especially elderly patients. Patients should be adequately hydrated and a check of renal function should be considered after the initiation of combination treatment and at regular intervals during treatment (see section 4.4).
Beta-blockers: NSAIDs counteract the antihypertensive effect of beta-adrenoceptor blocking drugs.
Selective serotonin re-uptake inhibitors (SSRIs):
SSRIs and NSAIDs each entail an increased risk of bleeding, e.g. from the gastrointestinal tract. This risk is increased by combination therapy. The mechanism may possibly be linked to reduced uptake of serotonin in the platelets (see section 4.4).
Cyclosporine: The concomitant administration of NSAIDs and cyclosporine is thought to be capable of increasing the risk of nephrotoxicity due to decreased synthesis of prostacyclin in the kidney. Accordingly, in the event of combination treatment, renal function must be monitored closely.
Captopril: Experimental studies indicate that ibuprofen counteracts the effect of captopril on sodium excretion.
Colestyramine: The concomitant administration of ibuprofen and colestyramine retards and reduces (by 25%) the absorption of ibuprofen. These drugs should be given at an interval of at least 2 hours.
Thiazides, thiazide-related preparations and loop diuretics: NSAIDs can counteract the diuretic effect of furosemide and bumetanide, possibly through inhibition of prostaglandin synthesis. They can also counteract the antihypertensive effect of thiazides.
Tacrolimus: Concomitant administration of NSAIDs and tacrolimus is thought to be capable of increasing the risk of nephrotoxicity due to decreased synthesis of prostacyclin in the kidney. Accordingly, in the event of combination treatment, renal function should be monitored closely.
Methotrexate: The risk of a potential interaction between an NSAID and methotrexate should also be taken into account in connection with low-dose treatment with methotrexate, especially in patients with renal impairment. Whenever combination treatment is given, renal function should be monitored. Caution should be exercised if both an NSAID and methotrexate are given within 24 hours, as the plasma levels of methotrexate can increase, resulting in increased toxicity (see above).
Corticosteroids: Concomitant treatment gives rise to an increased risk of gastrointestinal ulceration or bleeding.
Antiplatelet drugs: Increased risk of gastrointestinal bleeding (see above).
CYP2C9 Inhibitors: Concomitant administration of ibuprofen with CYP2C9 inhibitors may increase the exposure to ibuprofen (CYP2C9 substrate). In a study with voriconazole and fluconazole (CYP2C9 inhibitors) an increased S(+)-ibuprofen exposure by approximately 80 to 100% has been shown. Reduction of the ibuprofen dose should be considered when potent CYP2C9 inhibitors are administered concomitantly, particularly when high-dose ibuprofen is administered with either voriconazole or fluconazole.
Paracetamol:
Probenecid inhibits the binding of paracetamol to glucuronic acid, thus leading to a reduction in paracetamol clearance by a factor of approximately 2. In patients concurrently taking probenecid, the paracetamol dose should be reduced. Enzyme-inducing drugs such as certain antiepileptics (phenytoin, phenobarbital, carbamazepine) decreased plasma AUC of paracetamol to approximately 60% in pharmacokinetic studies. Other substances with enzyme-inducing properties (i.e. rifampicin, Hypericum) could also result in decreased concentrations of paracetamol. In addition, the risk of liver damage during treatment with the maximum recommended dose of paracetamol is probably higher in patients who receive enzyme-inducing drugs.
Zidovudine may affect paracetamol metabolism and vice versa, which may add to the toxicity of both.
Repeated paracetamol intake for longer than one week enhances the effects of anticoagulants, particularly warfarin. Therefore long-term administration of paracetamol in patients who are being treated with anticoagulants should only take place under medicinal supervision. The effect may occur already at daily doses of 1.5-2 g for 5-7 days. If paracetamol is used in doses at > 2 g daily, INR values (International Normalized Ratio) should be monitored. Occasional paracetamol intake has no significant effects on bleeding tendency.
Concurrent intake of medicinal products that accelerate gastric emptying, such as metoclopramide or domperidone, accelerates the absorption and onset of effect of paracetamol.
Cholestyramine may reduce the absorption of paracetamol and should therefore not be administered within an hour following paracetamol administration. Isoniazid may affect the pharmacokinetics of paracetamol with possible potentiation of liver toxicity.
Concurrent intake of medicines that slow gastric emptying can delay the absorption and onset of effect of paracetamol.
Paracetamol may affect the pharmacokinetics of chloramphenicol. Monitoring of chloramphenicol plasma levels is recommended if combining paracetamol with chloramphenicol injection treatment.
Ethyl alcohol potentiates paracetamol toxicity, possibly by inducing hepatic production of paracetamol-derived hepatotoxic products.
Effects on laboratory tests
Intake of paracetamol can affect tests for uric acid using phosphotungstic acid and blood sugar tests using glucose-oxidase-peroxidase.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Fertility, pregnancy and lactation
Pregnancy
For ibuprofen
Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/fetal development. Data from epidemiological studies suggest an increased risk of miscarriage and of cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation was increased from less than 1%, up to approximately 1.5 %. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre- and post-implantation loss and embryo-fetal lethality. In addition, increased incidences of various malformations, including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during the organogenetic period. During the first and second trimester of pregnancy, ibuprofen should not be given unless clearly necessary. If ibuprofen is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to:
• cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension);
• renal dysfunction, which may progress to renal failure with oligohydramnios; the mother and the neonate, at the end of pregnancy, to:
• possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses.
• inhibition of uterine contractions resulting in delayed or prolonged labour. Consequently, ibuprofen is contraindicated during the third trimester of pregnancy.
For paracetamol
A large amount of data on pregnant women indicate neither malformative, nor feto/neonatal toxicity. Paracetamol can be used during pregnancy if clinically needed however it should be used at the lowest effective dose for the shortest possible time and at the lowest possible frequency.
Breastfeeding
Paracetamol is excreted in breast milk but not in a clinically significant amount and available published data do not contraindicate breastfeeding.
Ibuprofen and its metabolites can pass in very small amounts into breast milk. No harmful effects to infants are known.
In light of the above evidences it is not necessary to interrupt breastfeeding, for shortterm treatment with the recommended dose of this product.
Fertility
The use of the product may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of the product should be considered.
4.7 Effects on ability to drive and use machines
In general, ibuprofen has no negative impact on the ability to drive or operate machinery. But because at high doses, side effects such as fatigue, vertigo ( reported as common) and visual disturbances ( reported as uncommon) can occur, the ability to drive and use machines may be impaired in some patients.
4.8 Undesirable effects
Clinical trials with Paracetamol/Ibuprofen have not indicated any other undesirable effects other than those for paracetamol alone or ibuprofen alone.
Adverse reactions have been ranked under headings of frequency using the following convention:
Very common (> 1/10);
Common (> 1/100 to < 1/10);
Uncommon (> 1/1,000 to < 1/100);
Rare (> 1/10,000 to < 1/1,000);
Very rare (< 1/10,000)
Not known (cannot be estimated from the available data).
Very rare cases of serious skin reactions have been reported
|
Blood and lymphatic system disorders |
Uncommon: Neutropenia, agranulocytosis, aplastic anaemia, haemolytic anaemia (sometimes Coombs positive), thrombocytopenia with or without purpura, leucopoenia, pancytopenia, eosinophilia and decrease in haemoglobin and haematocrit, epistaxis, menorrhagia. |
|
Immune system disorders |
Uncommon: Allergic Reactions: Syndrome of abdominal pain, fever, chills, nausea and vomiting, anaphylaxis, |
|
bronchospasm. Serum sickness, lupus erythematosus syndrome, Henoch-Schonlein vasculitis, angioedema. Very Rare: Hypersensitivity reactions, skin rash and crosssensitivity with sympathomimetics. | |
|
Metabolism and nutrition disorders |
Very Rare: metabolic acidosis, hypokalemia. Uncommon: Gynaecomastia, hypoglycaemic reaction |
|
Psychiatric disorders |
Very Rare: Confusion, depression, sleep disturbances, irritability, anxiety, restlessness, excitability. |
|
Nervous system disorders |
Common: Dizziness, headache, nervousness, vertigo, fatigue, agitation, irritability Uncommon: Depression, insomnia, confusion, emotional lability, somnolence, aseptic meningitis with fever and coma Rare: Paraesthesias, hallucinations, dream abnormalities Very Rare:,Paradoxical stimulation, optic neuritis, psychomotor impairment, extrapyramidal effects, tremor and convulsions. |
|
Eye disorders |
Uncommon: Amblyopia (blurred and/or diminished vision, scotomata and/or changes in colour vision) usually reversible on cessation of therapy. |
|
Ear and labyrinth disorders |
Common: Tinnitus |
|
Cardiac disorders |
Common: Oedema, fluid retention (usually reversible on discontinuation). Uncommon: arrhythmias (sinus tachycardia, sinus bradycardia) Very Rare: Palpitations; tachycardia; arrhythmia and other cardiac dysrhythmias. Hypertension and cardiac failure, hypotension. |
|
Respiratory, thoracic and mediastinal disorders |
Uncommon: Thickened respiratory tract secretions Very Rare: Asthma, exacerbation of asthma, bronchospasm and dyspnoea. |
|
Gastrointestinal Disorders |
Common: Abdominal pain, diarrhea, dyspepsia, nausea, stomach discomfort. Vomiting, constipation, abdominal cramps or pain, fullness of the GI tract (bloating and flatulence) Uncommon: Peptic ulcer, perforation or gastrointestinal haemorrhage, melaena, haematemesis sometimes fatal, particularly in the elderly. Ulcerative stomatitis and exacerbation of ulcerative colitis and Crohn's disease. Gastritis, pancreatitis. |
|
Hepatobiliary disorders |
Very Rare: Abnormal liver function, hepatitis and jaundice. Hepatic failure, hepatic necrosis and liver injury. |
|
Skin and subcutaneous tissue disorders |
Common: Rash (including maculopapular type), pruritus, angioedema and face swelling. Uncommon: Vesiculobullous eruptions, urticaria, erythema multiforme, alopecia, photoallergic skin reactions Very Rare: Hyperhidrosis, Exfoliative dermatoses, necrotising fasciitis. Bullous reactions including erythema multiforme. |
|
Renal and urinary disorders |
Uncommon: Urinary retention, oedema, nephrotic syndrome, |
|
interstitial nephritis. Very Rare: Nephrotoxicity, and acute and chronic renal failure. Acute tubular necrosis | |
|
General disorders and administration site conditions |
Very Rare: Fatigue and malaise. |
|
Investigations |
Common: Alanine aminotransferase increased, gamma-glutamyltransferase increased and liver function tests abnormal. Blood creatinine increased and blood urea increased. Uncommon: Aspartate aminotransferase increased, blood alkaline phosphatase increased, blood creatine phosphokinase increased, haemoglobin decreased and platelet count increased. |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
Paracetamol
Symptoms
In many cases of paracetamol overdosage there are often no early symptoms. Pallor, nausea, vomiting, anorexia and abdominal pain are early symptoms of paracetamol overdosage. Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema, and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, haematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Management
Due to differences in the management guidelines of paracetamol overdose in the different member states, as well as the continuous update of treatment recommendations, the national poison centre should be consulted for the most up-to-date treatment recommendations for the treatment of paracetamol overdose.
Immediate treatment is essential in the management of paracetamol overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention.
If acute paracetamol overdose or staggered paracetamol overdose is suspected, treatment with N-acetylcysteine should be started immediately.
Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour.
N-acetylcysteine should be dosed based on weight-based acetylcysteine dosing tables for adults and children found in the N-acetylcysteine SmPC or in the national treatment guidelines.
The duration of N-acetylcysteine treatment depends on the level of liver damage and should comply with national guideline recommendations.
Contact to a liver unit should be taken
• on signs of hepatocellulary damage (elevated ALAT) and critically affected coagulation parameters (INR > 1.6);
• Renal dysfunction and/or metabolic acidosis, that cannot be corrected by fluids;
• Encephalopathy
Ibuprofen
In children ingestion of more than 400 mg/kg may cause symptoms. In adults the dose response effect is less clear cut. The half-life in overdose is 1.5-3 hours.
Symptoms
Most patients who have ingested clinically important amounts of NSAIDs will develop no more than nausea, vomiting, epigastric pain, or more rarely diarrhoea. Tinnitus, headache and gastrointestinal bleeding are also possible. In more serious poisoning, toxicity is seen in the central nervous system, manifesting as drowsiness, occasionally excitation and disorientation or coma. Occasionally patients develop convulsions. In serious poisoning metabolic acidosis may occur and the prothrombin time/ INR may be prolonged, probably due to interference with the actions of circulating clotting factors. Acute renal failure and liver damage may occur. Exacerbation of asthma is possible in asthmatics.
Management
Management should be symptomatic and supportive and include the maintenance of a clear airway and monitoring of cardiac and vital signs until stable. Consider oral administration of activated charcoal if the patient presents within 1 hour of ingestion of a potentially toxic amount. If frequent or prolonged, convulsions should be treated with intravenous diazepam or lorazepam. Give bronchodilators for asthma.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Musculoskeletal system, anti-inflammatory and antirheumatic products, non- steroids, propionic acid derivatives. Ibuprofen combinations. ATC code: M01AE51
Mechanism of action
Although the exact site and mechanism of analgesic action of paracetamol is not clearly defined, it appears that it induces analgesia by elevation of the pain threshold. The potential mechanism may involve inhibition of the nitric oxide pathway mediated by a variety of neurotransmitter receptors including N-methyl-D- aspartate and substance P.
Ibuprofen is a propionic acid derivative with analgesic, anti-inflammatory and antipyretic activity. The drug's therapeutic effects as an NSAID result from its inhibitory effect on the enzyme cyclo-oxygenase, leading to reduction in prostaglandin synthesis.
The exact mechanism of action of ibuprofen is thought to be through peripheral inhibition of cyclooxygenases and subsequent prostaglandin synthesise inhibition.
Clinical efficacy and safety
Randomized, double-blind studies were conducted with the combination using the acute dental pain model of post-operative pain. The studies showed that:
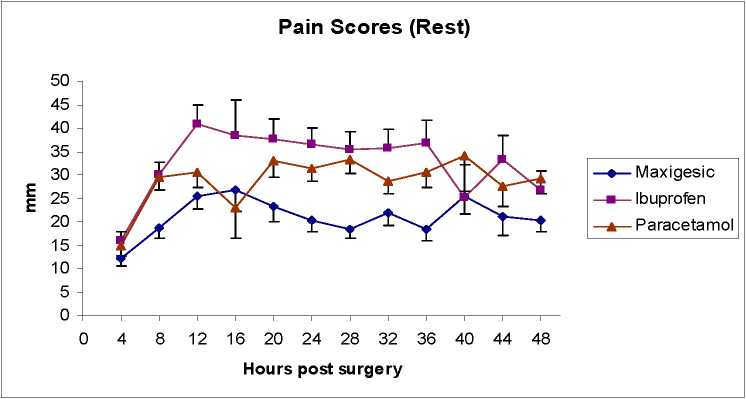
• Over the 48 hours, this product had a faster onset than either of its two active ingredients and provided superior analgesia than the same daily dose of paracetamol (At rest: p=0.007, 90% CI [mm]: -20.04, -4.10, On activity, p=0.006, 90% CI [mm]: -21.54, -4.58) and ibuprofen (At rest p=0.003, 90% CI [mm]: -21.54, -4.58, On activity, p=0.007, 90% CI [mm]:-22.09, 4.56).
Pain Score Plot-- Scores Given Are Those Rated During Each 4-Hour Period Post Surgery
• All three doses evaluated (half tablet or one tablet or two tablets) were effective when compared with placebo (Two tablets: p=0.004, 95% CI [mm/hr]:-23.32, -4.66; One tablet: p=0.002, 90% CI [mm/hr]:-20.78, -4.84; Half tablet: p=0.002, 95% CI [mm/hr]:-20.73, -4.51) and the highest dose [two tablets] had the greatest response rate (50%, p=0.003), lowest maximum VAS pain scores (p=0.009, 95% CI [mm]: -17.73, -2.41), longest time to rescue medication (p=0.001, 95% CI [hrs]: 2.27, 12.41) and lowest % of patients requiring rescue medication (53.3%, p=0.007). All these measures were significantly different to placebo (p<0.05).
Pain Intensity Differences by Treatment Group during the first six hours after first dose
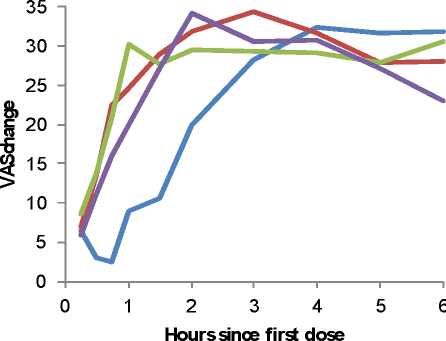
Placebo
Maxigesic 1/4 dose Maxigesic 1/2 dose Maxigesic Standard
Footnote: Maxigesic is Paracetamol/Ibuprofen in these figures
5.2 Pharmacokinetic properties
Absorption
Both paracetamol and ibuprofen are readily absorbed from the gastrointestinal tract with peak plasma concentration occurring about 10 to 60 minutes after oral administration.
Distribution
As for any product containing paracetamol, it is distributed into most body tissues. Biotransformation
Paracetamol is metabolised extensively in the liver and excreted in the urine, mainly as inactive glucuronide and sulphate conjugates. Less that 5% is excreted unchanged. The metabolites of paracetamol include a minor hydroxylated intermediate which has hepatotoxic activity. This active intermediate is detoxified by conjugation with glutathione, however, it can accumulate following paracetamol overdosage and if left untreated has the potential to cause severe and even irreversible liver damage. Paracetamol is metabolised differently by premature infants, newborns, and young children compared with adults, the sulphate conjugate being most predominant.
Ibuprofen is highly bound (90-99%) to plasma proteins and is extensively metabolised to inactive compounds in the liver, mainly by glucuronidation.
The metabolic pathways of paracetamol and ibuprofen are distinct and there should be no drug interactions where the metabolism of one affects the metabolism of the other. A formal study using human liver enzymes to investigate such a possibility failed to find any potential drug interaction on the metabolic pathways.
In another study, the effect of ibuprofen on the oxidative metabolism of paracetamol was evaluated in healthy volunteers under fasting conditions. The study results indicated that ibuprofen did not alter the amount of paracetamol undergoing oxidative metabolism, as the amount of paracetamol and its metabolites (glutathione-, mercapturate-, cysteine-, glucuronide- and sulfate-paracetamol) were similar when administered alone, as paracetamol, or with the concomitant administration of ibuprofen (as a fixed combination Paracetamol/Ibuprofen) This study clears any added hepatic risks from the hepatotoxic metabolite, NAPQI, from paracetamol if administered with Ibuprofen,
Elimination
Paracetamol elimination half-life varies from about 1 to 3 hours.
Both the inactive metabolites and a small amount of unchanged ibuprofen are excreted rapidly and completely by the kidney, with 95% of the administered dose eliminated in the urine within four hours of ingestion. The elimination half-life of ibuprofen is in the range of 1.9 to 2.2 hours.
Pharmacokinetic/pharmacodynamic relationship(s)
A specific study to investigate possible effects of paracetamol on the plasma clearance of ibuprofen and vice versa did not identify any drug interactions.
5.3 Preclinical safety data
The toxicological safety profile of ibuprofen and paracetamol has been established in animal experiments and in humans from extensive clinical experience. There are no new preclinical data of relevance which are additional to the data already presented in this Summary of Product Characteristics
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Pre-gelatinised maize starch Maize starch
Microcrystalline cellulose Croscarmellose sodium Magnesium stearate
Tablet coating: HPMC 2910/Hypromellose 15cP (E464), Lactose monohydrate, Titanium dioxide (E171), Macrogol/PEG-4000, Sodium citrate-dihydrate (E331)
Talc
6.2 Incompatibilities
Not applicable
6.3 Shelf life
3 years
6.4 Special precautions for storage
Store below 30°C.
Store in the original package in order to protect from light
6.5 Nature and contents of container
Pack sizes:
PVC/Al blisters containing 8, 10, 12, 16, 20, 24, 30 or 32 film-coated tablets. Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Actavis Group PTC ehf.
Reykjavikurvegi 76-78 220 HafnarfjorQur Iceland
8 MARKETING AUTHORISATION NUMBER(S)
PL 30306/0553
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
17/09/2015
DATE OF REVISION OF THE TEXT
17/09/2015
10