Prenoxad 1mg/Ml Injection
PACKAGE LEAFLET: INFORMATION FOR THE USER D02111
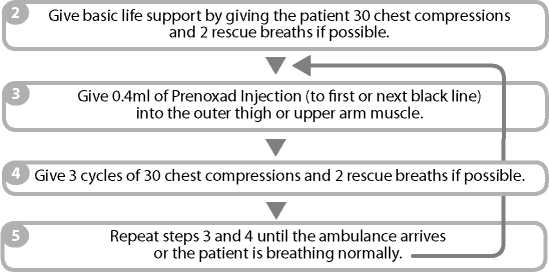
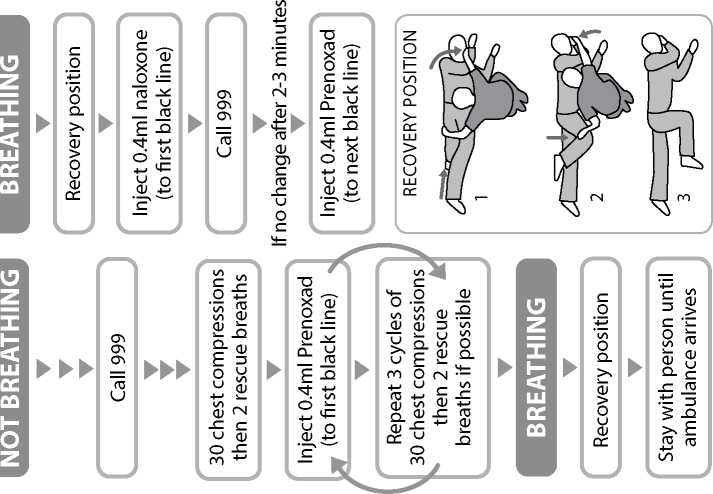
IF THE PATIENT DoES NoT APPEAR To BE BREATHING NoRMALLY: 1 Call 999 immediately a nd ask for an ambulance.
Naloxone Hydrochloride 1mg/1ml Solution for Injection
Because of your condition it may not be possible for you to read this leaflet before you are given Prenoxad Injection. The leaflet has been provided to you to give some information that you should have and to assist the person who is helping you.
You may wish to read it later.
• If you have any further questions, please ask your doctor or nurse
• If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or nurse
In this leaflet:
1. What Prenoxad Injection is and what it is used for
2. Before Prenoxad Injection is given
3. How Prenoxad Injection will be given
4. Possible side effects
5. Storing Prenoxad Injection
6. Contents of the Pack and other information
1. What Prenoxad Injection is and what it is used for.
Prenoxad Injection contains the medicine naloxone. Naloxone belongs to a group of medicines that reverse the action of opioid drugs e.g. diamorphine (heroin), methadone, dextropropoxyphene, nalbuphine, pentazocine.
This medicine is used to:
• reverse the action of opioid drugs e.g. if you have been given or taken an overdose of these drugs.
• If you are at risk of an opioid overdose you should always carry your Prenoxad Injection with you. It is designed as an emergency rescue treatment but you should still get medical attention as soon as possible.
2. Before Prenoxad Injection is given
This medicine is often used in circumstances where it is necessary to act very rapidly. You should not be injected with this medicine unless it is in the circumstances that were explained when you were given the Prenoxad Injection.
Prenoxad Injection will only be made available once the prescriber has assessed the suitability and ability of a client or a representative to administer naloxone in the appropriate circumstances You should not be given Prenoxad Injection if:
• you are allergic (hypersensitive) to Naloxone or to any other ingredients in this medicine, listed in section 6 of this leaflet.
Before giving you the Prenoxad Injection your prescriber will have considered whether special care needs to be taken if:
• you have kidney or liver problems
• you have heart problems
If you have any of these problems you should make your doctor aware or remind him. Taking other medicines
If you are able, you must tell your doctor if you are taking or have recently taken any medicines, including those obtained without a prescription. Pregnancy and breast-feeding
Prenoxad Injection should not be used if you are pregnant or breast-feeding unless it is absolutely essential.
Driving or using machines
Please speak to your doctor before driving or using machines.
Prenoxad Injection contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per 2ml dose, i.e. it is essentially 'sodium- free'
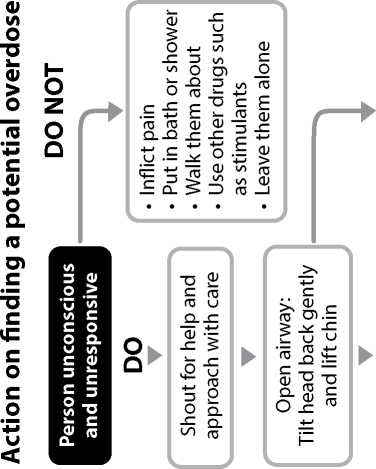
When the patient is breathing normally move them to the recovery position (lying on their side, mouth open and pointing towards the ground). Watch continuously.
If medical assistance has not arrived after you have used up the contents of one syringe and you have a second syringe available then this may be used using the same procedure as with the first. Use of the second syringe in the same way as the first does not present a safety hazard.
Prenoxad Injection is for single patient use only and any unused injection
solution should be discarded as instructed in section 5
If you are given more or less Prenoxad Injection than you should have
If you think you have been given too much or too little tell your doctor or the ambulance crew at the scene of the overdose.
3. How Prenoxad Injection will be given
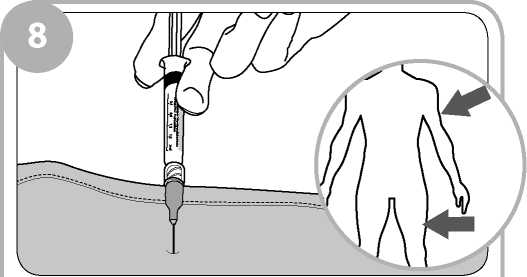
You or the person instructed by your doctor and clinic will give 0.4ml of the injection solution at any one time, into the outer thigh muscle or upper arm muscle (intramuscularly). The number of times injections of 0.4ml will be repeated will depend on your individual need and response to the treatment.
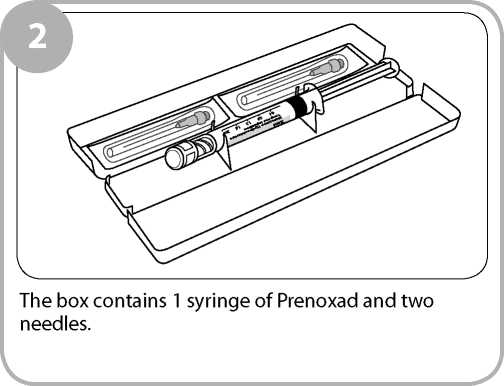
The pack contains two needles. The second needle is provided in case the first needle is damaged or gets contaminated because for example you have dropped it on the floor.
Adults
4. Possible side effects
Like all medicines Prenoxad Injection can cause side effects, although not everybody gets them.
Possible side effects include:
Very common (incidence >1 in 10): nausea
Common (incidence greater than 1 in 100 but less than 1 in 10): dizziness, headache, faster beating of the heart, increased blood pressure, vomiting. Uncommon (incidence greater than 1 in 1000 but less than in 100): tremor, sweating, irregular heartbeat, decreased heart rate, diarrhoea, dry mouth, local irritation, inflammation, faster or deeper breathing
Rare (incidence greater than 1 in 10000 and less than 1 in 1000): seizure, tension
Very rare (incidence less than 1 in 10000): allergic reactions (urticaria, rhinitis, dyspnoea, swelling, anaphylactic shock, cardiac arrest, redness of the skin with blisters or ulcers
Frequency unknown: fever, nervousness restlessness, irritability, runny nose, sneezing, yawning, piloerection, weakness, shivering, death
If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or nurse
5. Storing Prenoxad Injection
Keep out of the sight and reach of children.
You should not be given Prenoxad Injection after the expiry date which is printed on the box and syringe label. The doctor, nurse or pharmacist will check the expiry date on the box and syringe label before giving the pack to you.
The service that prescribed the product should be contacted so that they can advise you how to dispose of unused expired product or left over used product, including used and unused needles. Following use any left over product, including used and unused needles, may be given to the attending ambulance crew. Medicines should not be disposed of via drains or household waste.
Store in the original box to protect from light.
Do not store above 25°C. If the injection is discoloured it should not be used.
Known or suspected opioid overdose:
Prenoxad Injection should only be given where it is known or suspected that an opioid overdose has occurred.
What Prenoxad Injection contains.
The active substance is Naloxone Hydrochloride 1mg per ml.
The other ingredients are Sodium Chloride, Water for Injection and Dilute Hydrochloric Acid.
The following procedure should be followed:
See overleaf for diagrams to help the person give the injections.
IF THE PATIENT IS BREATHING NoRMALLY BUT IS UNRoUSABLE or UNCoNSCIoUS:
What Prenoxad Injection looks like and contents of the pack.
The injection is supplied in a 2ml prefilled syringe containing 2ml of a clear, colourless solution together with two needles. The syringe and needles are contained in a yellow box.
i
Move the patient into the recovery position, lying on their side, mouth open and pointing towards the ground.
M
Give 0.4ml of Prenoxad Injection (to first black line) into the outer thigh or upper arm muscle.
Marketing Authorisation Holder:
Aurum Pharmaceutical Ltd Bampton Road Harold Hill Romford, RM3 8UG United Kingdom
Manufacturer:
Martindale Pharmaceuticals Ltd.
Bampton Road
Harold Hill
Romford, RM3 8UG
United Kingdom
Product Licence Number: PL 12064/0125 Date of last revision: November 2012
Call 999 and ask for an ambulance.
Instructions for administration to patients may be found overleaf

M
Instructions for patient administration
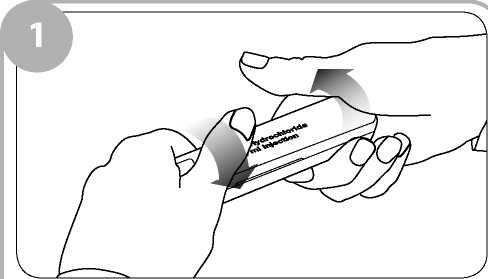
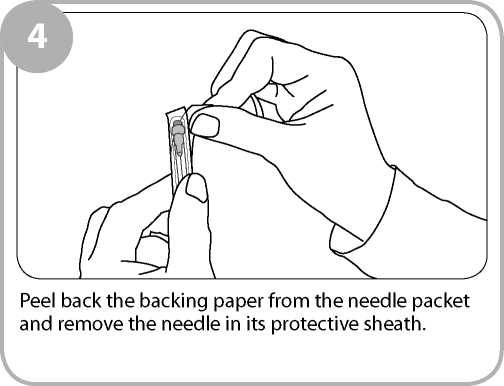
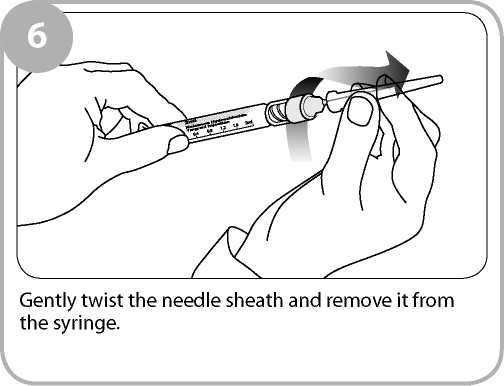
Remove the clear film wrapping by pulling the tear strip on the side of the box. Twist the outer plastic box as shown to break the tamper evident seals and open.
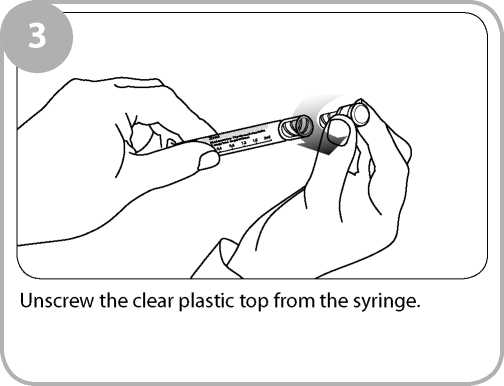
\a<
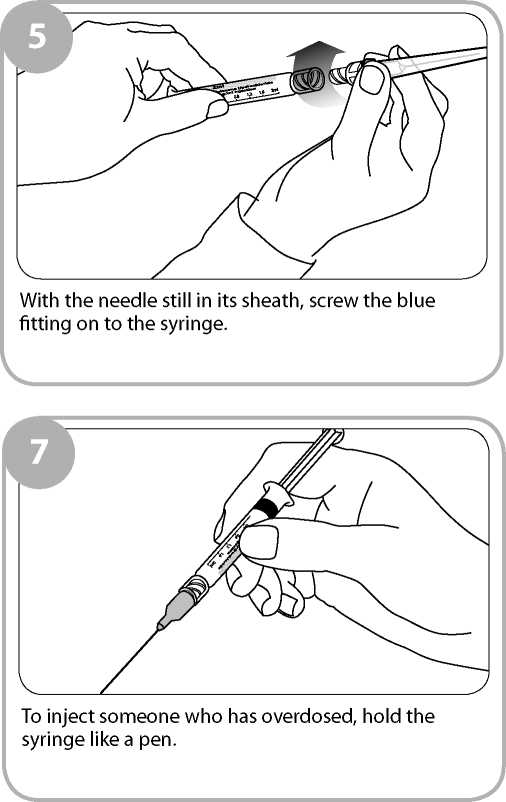
Insert the needle into the patient's outer thigh or upper arm, through clothing if necessary, and inject first dose (0.4ml). Withdraw the needle and syringe after each dose.
D02111
MARTJNDALE PHARMA
Component Code: D02111
Paper size: 200 x 400mm
|
Version Control |
Date |
By |
|
Version A Created |
17/02/12 |
AC |
|
Version B |
22/02/12 |
AC |
|
Version C |
05/04/12 |
AC |
|
Version D |
18/04/12 |
HM |
|
Version E |
26/04/12 |
AC |
|
Version F |
26/04/12 |
AC |
|
Version G |
21/05/12 |
AC |
|
Version H |
22/05/12 |
AC |
|
Version I |
28/05/12 |
AC |
|
Version J |
31/05/12 |
AC |
|
Version K |
11/06/12 |
AC |
|
Version L |
14/06/12 |
AC |
|
Version M |
28/06/12 |
AC |
|
Version N |
28/06/12 |
AC |
|
Version o |
01/10/12 |
AC |
|
Version P |
30/10/12 |
AC |
|
Version Q |
31/10/12 |
AC |
|
Version R |
31/10/12 |
AC |
|
Version S |
02/11/12 |
AC |
|
Version T |
16/11/12 |
AC |
|
Version U |
23/11/12 |
AC |
|
Version V |
26/11/12 |
AC |
|
Version W |
27/11/12 |
AC |
|
Version X |
03/12/12 |
AC |
|
Version Y | ||
|
Version Z |
Grand Fromage Creative ltd
60 Churchill Square, Kings Hill
West Mailing, Kent ME19 4YU t:+44 (0)1732 54 34 94 f:+44 (0)1732 54 34 04 e:studio@grand-fromage.co.uk